Dispensing Controlled Drugs
Published on: 5th July 2013 | Updated on: 18th April 2024
This page contains detailed information on dispensing Controlled Drugs (CDs), including regulations, instalment dispensing, methadone dispensing and reimbursement arrangements.
Controlled Drug (CD) prescription writing requirements:
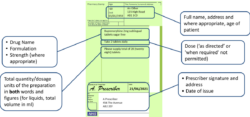
To be valid, in addition to the normal prescription requirements for Prescription Only Medicines (as required by the Human Medicines Regulations 2012), prescriptions for Schedule 2 and 3 CDs must also contain the following (as outlined in The Misuse of Drugs Regulations 2001):
- Full name, address and where appropriate, age of patient
- Drug name
- Formulation
- Strength (where appropriate)
- Dose (‘as directed’ or ‘when required’ not permitted)
- Total quantity/dosage units of the preparation in both words and figures (for liquids, total volume in ml)
- Prescriber signature and address
- Date of issue
- For instalment prescriptions, specify the instalment amount AND instalment interval
- The words “for dental treatment only” written on it if issued by a dentist
Medicines Ethics and Practice, published annually by the Royal Pharmaceutical Society, provides further and more detailed guidance on these requirements.
The prescription must be written in indelible ink and may be computer-generated (except for the prescriber’s signature which must be handwritten unless the prescription is issued via EPS). For patient address, the use of PO Box is not acceptable; However, use of No Fixed Abode as an address is acceptable for example in the case of homeless patient. The dosage form for example tablets, capsules etc. must be included on a CD prescription irrespective of whether it is implicit in the proprietary name, or whether only one form is available. Where appropriate, the strength of the drug must be included (when more than one strength of a preparation exists the strength required must be specified). The dose, which must be clearly defined (i.e. the instruction ‘one as directed’ constitutes a dose but ‘as directed’ on its own does not).
Total quantity of preparation must be expressed in both words and figures. For liquids, the total volume in millilitres (in both words and figures) of the preparation to be supplied; for dosage units such as tablets, capsules, ampoules) the total number (in both words and figures) of dosage units to be supplied must be specified (e.g. 28 (twenty eight) tablets. The prescription must be signed by the prescriber with their usual handwritten signature – Advanced electronic signatures can be accepted for Schedule 2 and 3 CDs where EPS is used. The prescription must specify the prescriber’s address (which must be within the UK). The prescription must include the date on which it was signed/issued – a computer-generated date or rubber stamp is acceptable. The words “for dental treatment only” must be present if the prescription is issued by a dentist. For instalment prescriptions, the prescription must specify the instalment amount AND instalment interval.
Drugs that are not CDs should not be prescribed on the same form as a Schedule 2 or 3 CD.
Length of treatment:
The Department of Health and Social Care (DHSC) has issued strong recommendations that prescriptions for Schedule 2, 3 and 4 CDs are limited to the quantity necessary for up to 30 days’ treatment. Exceptionally, where a prescriber believes that a prescription for a CD should be issued for a longer period he/she may do so where there is a genuine clinical need and it does not pose an unacceptable risk to patient safety. Pharmacists can dispense prescriptions ordering more than 30 days’ supply of any Schedule 2, 3 and 4 CDs.
Prescription validity:
Prescriptions for Schedule 2, 3 and 4 CDs are only valid for 28 days after the appropriate date (i.e. date of signing unless the prescriber indicates a date before which the CD should not be dispensed). Note: a prescriber may forward-date a CD prescription in which case the date of validity is 28 days after the forward-date, or the start date, where specified.
For example, if the appropriate date is 1 November, the 28-day validity period would run until 29 November. Supply on 30 November would not be compliant with the regulations as the 28-day validity period would have ran out.
For FP10MDA prescriptions, the first instalment must be dispensed within 28 days of the appropriate date and the remainder should be dispensed in accordance with the directions on the prescription.
Prescriptions for Schedule 5 CDs are valid for dispensing for 6 months from the appropriate date. For further information on prescription validity, click here.
Owings:
In the case of owings, any remaining balance of Schedule 2, 3 or 4 CDs must be dispensed within 28 days of the appropriate date on the prescription. It is good practice for the pharmacist to make the patient or their representative aware from the outset that they will not be able to receive a supply of any prescribed Schedule 2, 3 or 4 CDs beyond the 28 day period of prescription validity.
For prescriptions for Schedule 5 CDs, the balance of an owing cannot be collected more than 6 months after the appropriate date.
Repeat dispensing:
Schedule 2 and 3 CDs cannot be prescribed on repeat dispensing prescriptions. Only Schedule 4 and 5 CDss are permitted on repeatable prescriptions.
Repeat dispensing prescriptions for Schedule 4 CDs must be dispensed for the first time within 28 days of the appropriate date. After the first dispensing episode is complete, the repeats are legally valid within the normal periods of validity of the repeatable prescription.
Repeat prescriptions for Schedule 5 CDs must be dispensed for the first time within six months of the appropriate date. After the first dispensing episode is complete, the repeats are legally valid within the normal periods of validity of the repeatable prescription.
Installment Dispensing:
For ‘blue’ FP10MDA prescriptions, the first instalment must be dispensed within 28 days of the appropriate date and the remainder should be dispensed in accordance with the directions on the prescription. The prescription must be marked with the date of each supply. The instalment direction is a legal requirement and needs to be complied with. However, for certain situations (e.g. if a pharmacy is closed on the day an instalment is due), the Home Office has approved specific wording which provides pharmacists some flexibility for making a supply. Note: there remains no provision to dispense CDs in instalments on a standard paper FP10 prescription form or via EPS.
EPS prescriptions:
Prescribers can issue EPS prescriptions for all Controlled Drugs (CDs) including Schedule 2 and 3 CDs but are currently unable to issue instalment ‘blue’ FP10MDA prescriptions electronically).
A method of “marking” the prescription “at the time of supply” should be decided to ensure compliance with the Misuse of Drugs Regulations 2001. The dispense notification message may be suitable for this but depending on local system configuration, another patient medication record (PMR) process that records a date and leaves an electronic audit trail may be suitable.
The 28-day validity period to supply a CD is different from the 180-day EPS technical time limit for sending dispense and claim notification messages for reimbursement and EPS technical purposes. Note: Some patient medication record (PMR) systems may refer to item/prescription ‘expiry’ , validity period or the 180-day EPS technical time limit.
Read more at EPS CDs webpages, EPS CDs one-page factsheet and Controlled Drug resources and FAQs.
Amending typographical errors on paper prescriptions:
Pharmacists are able to amend prescriptions for Schedule 2 and 3 CDs where the prescription does not comply with the CD prescription requirements. The only changes that pharmacists can make are:
- minor spelling mistakes; or
- minor typographical mistakes (this may include, for example, a number being substituted for a letter or two letters being inverted but where the prescriber’s intention is still clear); or
- where the total quantity of the CD/number of dosage units is specified in either words or figures but not both, a pharmacist can add either the missing words or figures as required (but not both).
In doing this, the pharmacist must exercise due diligence and be satisfied that the prescription is genuine and the CD is being supplied in accordance with the prescribers’ intentions. The prescription must be amended in ink or otherwise indelibly and the pharmacist must mark the prescription so that the amendment is attributable to him or her (e.g. name, date, signature, and GPhC registration number). If there is more than one amendment on the same prescription, each amendment must be countersigned.
Where an amendment is made by one pharmacist and another pharmacist makes the supply, the Home Office has advised that the second pharmacist should also mark the amendment to indicate that he is also satisfied and it is attributable to him as well.
Identity checks:
When a Schedule 2 CD is collected from a pharmacy, legislation states that the pharmacist must ascertain whether the person collecting is the patient, patient’s representative or healthcare professional. In the case of Schedule 2 CD prescriptions (for example, methadone), the person may, if not already known to the pharmacist, be asked to present some form of identification. If the person collecting the Schedule 2 CD is a healthcare professional acting in their professional capacity on behalf of the patient, the pharmacist must ask for identification and obtain evidence of the name, address and professional registration number of the healthcare professional. Note that the requirements for signing the prescription and identification on collection apply to instalment prescriptions only on the first occasion that the patient presents. Where the person collecting the CDs refuses to provide their details (e.g. the name to record in the PMR or does not sign the reverse of the form/token), a pharmacist, using their professional discretion, may still supply the CDs.
As with any other NHS prescription, the reverse should be completed in full with the patient’s declaration of payment or exemption status and their signature. A representative, including a delivery driver, can sign on behalf of a patient. However, a robust audit trail should be available to confirm successful delivery of the medicine to the patient. Instalment prescriptions only need to be signed once. Alternatively, some pharmacists may wish to record details of the CD collector electronically (e.g. within the patient’s record). Retaining electronic records within the pharmacy and reducing use of paper tokens to capture signatures of CD collectors helps to align with the NHS’s paperless objectives.
It is good practice for the person collecting a Schedule 2 or 3 CD to sign the reverse of the prescription form or EPS dispensing tokens (FP10DT) which has a space for the ‘Signature of collector of Schedule 2 & 3 CDs’ that is specifically for this purpose. A supply can still be made if this is not signed, subject to the pharmacists professional judgement.
If a patient pays for their prescriptions, they would only need to pay one prescription charge for each item. On a FP10MDA form, patients do not pay a charge for each instalment collected. However, if two different items (for example buprenorphine and diazepam) or an item with two different formulations of same drug (caps and tabs) are ordered on a FP10MDA form, two prescription charges would apply.
For information on record-keeping requirements click here.
Collectors of CDs signing the back of prescription forms or tokens:
Best practice guidance to record the details of the person collecting a Schedule 2 or 3 CD remains in place; the reverse of NHS prescription forms and EPS dispensing tokens (FP10DT) have a box for the ‘Signature of a collector of Schedule 2 & 3 CDs’ which can be used to obtain a signature. Any tokens used to collect a signature can be sent to the NHS Business Services Authority (NHSBSA), as appropriate. Alternatively, some contractors may wish to record details of the CD collector electronically (e.g. within the patient’s record). Retaining electronic records within the pharmacy and reducing the use of paper tokens helps to align with the long-term NHS paperless objectives.
Where the person collecting the CDs refuses to provide their details (e.g. the name to record in the PMR or not signing the reverse of the form/token), pharmacists may apply their discretion on whether or not to supply the CDs.
When the CD is supplied, it is a requirement to mark the prescription with the date of supply at the time the supply is made. With EPS, that marking may be done automatically (see EPS prescriptions section above).
Private prescriptions for Schedule 2 & 3 CDs:
In England, prescribers can now only order Schedule 2 or 3 CDs privately on a specially designated private prescription form FP10PCD. In Wales, the required form is WP10PCD or WP10PCDSS and in Scotland, Form PPCD (1). This requirement does not extend to Veterinary prescriptions.
Prescriber identification number:
All private prescribers have been allocated a six-digit prescriber identification number (issued by the relevant NHS agency) which must be included on private form FP10PCD (or the Welsh or Scottish equivalent). Private prescriptions for Schedule 2 or 3 CDs should not be dispensed in community pharmacies if they do not contain this identifier. Private prescribers should be referred to their primary care organisation (e.g. local NHS England team) if they require a private prescriber identification number.
Submission:
The Misuse of Drugs Regulations 2001 was amended in 2006 to introduce a requirement for all private prescriptions for Schedule 2 and 3 CDs to be sent to the NHS Prescription Services or its equivalent body for analysis and monitoring purposes.
Amendments to the medicines legislation (the Medicines for Human Use (Administration and Sale or Supply)(Miscellaneous Amendments) Order 2007 and the Medicines (Sale or Supply) (Miscellaneous Provisions Amendment Regulations 2007) have been made to allow for the original private FP10PCD prescriptions to be sent to NHS Prescription Services and came into effect on the 1st September 2007.
Pharmacy contractors in England are required to submit FP10PCD forms to NHS Prescription Services for audit purposes each month using a special submission document, FP34PCD* which is available on the NHS Prescription Services website or alternatively, pharmacy teams may telephone or email NHS Prescription Services on 0300 330 1349 or nhsbsa.prescriptionservices@nhsbsa.nhs.uk to receive another form. The equivalent forms originating from Wales or Scotland should also be submitted.
Community pharmacies require a private CD account number which should be used when submitting FP10PCD private forms which is a different account to the NHS account number used by contractors to submit NHS prescriptions. In England, suppliers who need to submit private prescription forms but who do not already have a private CD prescription F code must contact their local NHS England team.
Pharmacy contractors in Wales should also submit the original private prescription form WP10PCD to Health Solutions Wales on a monthly basis at the same time as submitting other NHS prescription forms. The equivalent forms originating from England or Scotland should also be submitted.
This section contains information including regulations for Controlled Drugs (CDs), prescription validity, length of treatment, owings and private prescriptions for Schedule 2 & 3 CDs (submission).
In England, only the following can be supplied in instalments against form FP10MDA:
- Schedule 2 Controlled Drugs;
- Buprenorphine;
- Buprenorphine/naloxone (Suboxone); and
- Diazepam
In Wales, Schedule 2, 3, 4 and 5 Controlled Drugs can be supplied in instalments on form WP10MDA.
Both FP10MDA and WP10MDA forms are limited to a maximum period of treatment of 14 days. The prescriber must specify the instalment amount AND the interval between each instalment.
Supervised consumption:
As part of the approach to support patients undergoing opiate substitution therapy (OST), community pharmacies provide supervised consumption of methadone and buprenorphine as part of a locally commissioned service through either the Local Authority or via a subcontracted provider of shared care services
Supervised consumption is not a legal requirement under the Misuse of Drugs 2001 Regulations. However, where supervision is directed on a prescription, and this instruction is not followed, a pharmacist should consider the risks of supplying the drug unsupervised.
Where the instruction to supervise is not followed, It is legally acceptable to confirm verbally with the prescriber that they are happy with this arrangement since supervision, while important, is not a legal requirement under the 2001 Regulations. The Department of Health recommends that any deviation from the prescriber’s intended method of supply should be documented, and the justification for this recorded.
It is also good practice for pharmacists to alert the prescriber whenever there are significant concerns: such as when consecutive daily doses of OST have been missed, following repeated missed pick-up of single days (or even one missed day for certain patients) and when the pharmacist has any concerns regarding the patient’s presentation (such as intoxication or apparent significant deterioration in health and wellbeing)
Endorsing:
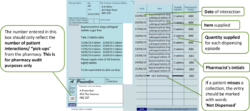
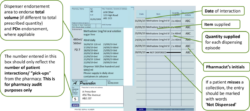
Pharmacists should use the right-hand side of the prescription to endorse with the volumes dispensed at each “pick-up” episode (i.e. each time the patient collects their drug from the pharmacy). No other endorsement is required.
Community Pharmacy England has produced guidance on how to endorse FP10MDA prescriptions (other than those prescribing oral liquid methadone): Community Pharmacy England’s FP10MDA endorsing guidance
How to endorse FP10MDAs (non-liquid oral methadone) correctly
How to endorse liquid oral methadone prescriptions correctly:
Pharmacists will endorse the prescription with the volumes dispensed at each “pick-up” episode (i.e. each time the patient collects their drug from the pharmacy).
No endorsement is required to be paid for each “pick-up” or “item level fee”, these are paid automatically for all prescriptions for oral liquid methadone (including FP10 forms).
An additional “Packaged Dose” fee of 55p can be claimed per additional bottle of oral liquid methadone supplied. The number of additional packaged doses claimed must be clearly endorsed on the prescription as payment of this fee will be based on the endorsement given only. The required endorsement is PD and the number of additional packaged doses supplied, e.g. PD 8 (see worked example that follows). The number of additional packaged doses supplied is equal to the total number of days’ supply given minus the number of patient interactions.
Blue FP10MDA
The number of additional packaged doses claimed must be clearly endorsed on the prescription as payment of this fee will be based on the endorsement given only. The number of additional packaged doses supplied is equal to the total number of days’ supply given minus the number of patient interactions. It must be endorsed as PD n (where n = Total number of separately packaged doses supplied minus Total number of patient interactions) as any other variation will not be accepted for payment. Most EPS systems now allow PD endorsement to electronic prescriptions for methadone oral solution.
Community Pharmacy England has produced a full guide of worked examples for several differing scenarios showing how to endorse FP10 and FP10MDA prescriptions for oral liquid methadone:
Community Pharmacy England’s methadone endorsing guidance
Sorting and submission:

The images above show how FP10MDA forms should be submitted. Before submission, pharmacy staff involved in end of month submission processes should check that the reverse of the MDA forms are correctly signed before sorting into their relevant exempt or paid groups.
The blue forms should be unfolded and banded together separating them from the rest of the prescription bundle.
Submitting the FP10MDA forms folded may slow down NHSBSAs prescription scanning processes.
If a FP10MDA is torn i.e. perforated section is broken, the form should be taped back together and place with the other FP10MDA forms.
FP10MDA forms should be placed together in the prescription bundle so they are easily located and can be looked at separately by a Pricing Authority exception handler.
Further information can be found on the ‘Sorting your prescriptions prior to submission‘ page.
Schedule of Payments:
The FP34 Schedule of Payments contains a section headed “Prescription Fees” and it is within this section that fees relating to oral liquid methadone prescriptions for that dispensing month can be found.
Payment is based on each occasion the pharmacist provides an instalment to the patient; i.e. each time the patient collects their drug from the pharmacy.
Below is a summary of the fees which are payable against a prescription for Controlled Drugs including oral liquid methadone:
| Applicable fee | Endorsement requirement | Payment |
| Single Activity fee (£1.27) | Each interaction must be endorsed with the total volume supplied (and initialed) for each patient interaction/ “pick-up” for FP10MDAs. | Paid per interaction or “pick-up” for FP10MDAs |
| Controlled Drug fee (£1.28) | ||
| Consumable allowance (£0.124) | ||
| Payment for containers (£0.10) | None required | Paid based upon total quantity prescribed (see below) |
| Additional fees for oral liquid methadone prescriptions | ||
| Applicable fee | Endorsement requirement | Payment |
| Item level fee £2.50 per prescription | None required | Paid once automatically per prescription (FP10MDA or FP10) for oral liquid methadone |
| Packaged Dose fee £0.55 per additional dose packaged separately (oral liquid methadone only) | Total number of separately packaged doses supplied minus total number of patient interactions | Paid based upon contractor endorsement |
For each interaction with the patient or “pick-up” for items prescribed on an FP10MDA form, contractors will receive a Single Activity Fee (SAF), Controlled drug fee, the consumables allowance and any relevant volume related fees. Where applicable, a payment for containers (split pack fee) of 10p will also be paid where the total quantity prescribed on the form (i.e. the sum of all doses) is outside of a pack size (or a multiple of the pack sizes) listed in Part VIIIA of the Drug Tariff.
This page contains information on the Controlled Drug Regulations and their implications for pharmacy practice.
Destruction of Patient Returned and Stock Controlled Drugs:
Pharmacy contractors must have appropriate arrangements in place for securing the safe destruction and disposal of Controlled Drugs (CD / CDs). The Royal Pharmaceutical Society (RPS) has issued guidance that unwanted CDs returned from patients homes in Schedule 2, Schedule 3 and Schedule 4 (part 1) should be placed into waste containers only after the CD has been rendered irretrievable (i.e. by denaturing). Where practicable pharmacists should use CD denaturing kits in order to denature CDs, these can be obtained from some waste contractors and the NPA. Where this is not possible other methods of denaturing may be used for example adsorbing onto cat litter, provided that there are no risks to human health or the environment. Detailed guidance on the disposal of unwanted medicines can be found in the waste section of this website.
Patient returned CDs: Pharmacies can accept CDs returned by patients from their own homes and from care homes providing personal care for safe destruction and onward disposal. Pharmacies in England and Wales are not able to accept waste medicines, including CDs, from care homes which provide nursing care for disposal under the NHS funded unwanted medicines service. The RPS guidance to Pharmacists is that patient returned schedule 2 CDs should be recorded and their subsequent destruction recorded (in a separate record to the CD register). Patient returned CDs should be denatured in the presence of another member of staff, preferably a pharmacist or pharmacy technician if available. RPS guidance confirms that the destruction of patient returned CDs, whether they require denaturing or not, does not require witnessing by an authorised person.
Date expired pharmacy stock: It is a legal requirement under the 2001 regulations for pharmacy contractors to have stocks of obsolete, expired and unwanted Schedule 1 and 2 CDs destroyed in the presence of an authorised witness. RPS guidance indicates that for Schedule 3 CDs it would be good practice to have another member of staff witness the denaturing.
An amendment to the Misuse of Drugs Regulations 2001 which came into force on the 16th August 2007 allows Accountable Officers to authorise people or groups of people (the authorised witness) to witness the destruction of controlled drugs to render them irretrievable. Previously the witnessing of destruction of controlled drugs was undertaken by the police chemist inspection officers, GPhC (formerly Royal Pharmaceutical Society) inspectors, and the Home Office inspectors. The authorised witness is a person who is not involved in the day-to-day handling of controlled drugs who has been appointed by the CDAO to oversee the management and governance of activities related to controlled drugs.
Multiples may be able to obtain authorisation from NHS England’s Lead Controlled Drugs Accountable Officer (CDAO), for specified persons to be the authorised witness to be present to confirm the destruction of CDs within the pharmacy business. The authority is not available to persons who would normally handle CDs in the course of their employment; but could be for example, regional managers. You may wish to contact your local NHS team to discuss with the CDAO.
Bodies corporate, partnerships and/or individuals operating fewer than 5 community pharmacies may not have suitably trained individuals designated as Authorised Witnesses. To facilitate the timely destruction of obsolete CDs, NHS England will designate sufficient, suitably trained, individuals and/or class of person as Authorised Witnesses. This may include NHS England employees and/or agents, representatives of the appropriate Local Pharmaceutical Committee and/or persons nominated as appropriate to undertake the role by a recognised trade association such as the National Pharmacy Association or Independent Pharmacy Federation.
An independent community pharmacy may also contract with an independent Authorised Witness to facilitate the witnessed destruction of obsolete CDs. Where an independent community pharmacy requests an Authorised Witness to witness the destruction of obsolete CDs from NHS England, NHS England will ensure that one is made available
Any person authorised to witness the destruction of CDs by the accountable officer should have the appropriate training and will be accountable for this directly to the CDAO.
CQC maintain a record of the details of healthcare organisations’ accountable officers in an online register, which is updated whenever CQC are notified of changes.
In November 2013, NHS England published guidance on the supervision of management and use of CDs. In April 2016, NICE published guidance on “Controlled drugs: safe use and management” which covers systems and processes for using and managing CDs safely in all NHS settings except care homes. It aims to improve working practices to comply with legislation and have robust governance arrangements. It also aims to reduce the safety risks associated with CDs.
NICE Guidance states that when destroying and disposing of pharmacy own stock CDs in Schedule 2 the following must be entered into the CD register:
- the name, strength and form of the controlled drug;
- the quantity;
- the date of destruction;
- the signature of the authorised person witnessing the destruction
There is no requirement in the 2001 regulations that the disposal of date-expired medicines in Schedules 3, 4 and 5 to be witnessed or recorded. Nevertheless, NICE guidance confirms that even if legislation does not require a witness to be present when destroying pharmacy own stock CDs to consider having a witness present.
Monitoring and Inspection Arrangements:
New arrangements were introduced in January 2007 for monitoring the management, usage and other aspects of controlled drugs in England. Separate Regulations which came into force in January 2009 govern the monitoring and inspection arrangements in Wales.
Every healthcare organisation (e.g. local NHS England team) must have an appointed ‘Accountable Officer’ with a duty to oversee the management and use of controlled drugs. The accountable officers appointed by local NHS England team have a range of responsibilities including ensuring that pharmacies have adequate and up-to-date standard operating procedures (SOPs) in relation to the use of CDs, have appropriate arrangements in place for securing the safe destruction and disposal of CDs and systems in place to alert the Accountable Officer of complaints or concerns involving the management and use of CDs.
As part of the new arrangements, the GPhC ask pharmacy contractors to make a declaration in relation to the management and use of CDs at each of their premises – this forms part of the annual premises retention fee cycle. Pharmacy contractors can make a bulk declaration for all of their premises if they own more than one.
SOPs: Pharmacy contractors are required under their terms of service to have SOPs for dispensing and repeat dispensing. The regulations now also require SOPs relating to the management and use of controlled drugs to cover the following points:
- Ordering and receipt of CDs;
- Assigning responsibilities;
- Where the Controlled Drugs are stored
- Who has access to the CDs
- Who should be alerted if complications arise
- Security in relation to the storage and transportation of CDs as required by the Misuse of Drugs regulations
- Disposal and destruction of CDs
- Record keeping, including maintaining relevant CDRs under the Misuse of Drugs legislation and maintaining a record of the CDs specified in Schedule 2 to the Misuse of Drugs Regulations 2001 that have been returned by patients
NB: Schedule 2 to the Misuse of Drugs Act 1971 deals with Class A, B and C “controlled drugs” which should not be confused with the 5 Schedules to the Misuse of Drugs Regulations – which pharmacy teams will be familiar with.
Gabapentin and Pregabalin. In October 2018, the Misuse of Drugs Act 1971 (Amendment) Order 2018 was laid before Parliament and will come into force on 1 April 2019. This change means that it will be illegal to possess pregabalin and gabapentin without a prescription and it will be against the law to supply or sell them to others. These drugs will become Schedule 3 CDs but not be subject to the safe custody requirements.
Monitoring and Supporting Staff Handling CDs: Pharmacy contractors need to have in place arrangements for monitoring and auditing the management and use of CDs by pharmacists and staff. The arrangements are required to include:
- Recording any concerns raised in relation to the management and use of CDs by the relevant individual;
- Assessing and investigating any concerns raised regarding the management and use of CDs by the relevant individual; and
- Determine if there are any concerns in relation to the management and use of CDs by a relevant individual which the pharmacy contractor considers should be shared with a responsible body e.g. GPhC
Pharmacy contractors must also ensure that pharmacists and staff handling CDs receive from time to time, appropriate training to carry out their responsibilities in the management and use of CDs.
Recording concerns: Pharmacy contractors should record concerns expressed about incidents that involved or may have involved improper management or use of CDs by a pharmacist or member of staff. The record should include the following:
- The date on which the concern is made known;
- The dates on which the matters giving rise to the concern;
- Details regarding the nature of the concern;
- Details of the pharmacist or staff involved;
- Details of the person or body raising the concern;
- Details of any action taken;
- An assessment of whether information should be disclosed to a responsible body; and
- If disclosure to a responsible body takes place, details of the disclosure and the responsible body to which the disclosure was made
An Accountable officer and police officers would have the power to enter and inspect pharmacy premises if concerns were being investigated.
Further guidance on the details required in the SOPs for CDs can obtained from the RPS and templates for CD SOPs may be obtained from the NPA.
Reporting CD related incidents:
Pharmacy teams should ensure that there are robust processes for reporting CD-related incidents.
NICE guidance indicates that when multiple systems are used for reporting controlled drug-related incidents (for example, local and national systems and occurrence reporting), consider developing a local process that coordinates these systems within the organisation. This may include: reviewing arrangements regularly to reflect local and national learning carrying out risk assessments of incidents sharing learning. It goes on to suggest that consideration be given to include in local processes for reporting controlled drug-related concerns or incidents: how to inform the controlled drugs accountable officer or nominated person reporting incidents in a timely way, ideally within 48 hours.
Record Keeping Requirements
Running Balances: As a matter of good practice pharmacists who supply CDs should maintain a running balance of stock in their Controlled Drug Register (CDRs). This is expected to become a mandatory requirement once electronic registers are in common use. Further guidance on the maintenance of a running balance in the CDR is available on the RPS Website (for members).
Controlled Drug Registers: A Controlled Drugs Register (CDR) must be used to record details of any Schedule 1 and Schedule 2 CDs received or supplied by a registered pharmacy. The 2001 regulations also require that additional information should be recorded in the CDR in relation to the identity of the person collecting a schedule 2 CD supplied on prescription.
The 2001 regulations require the following information to be recorded in the CDR, under the following specific headings, when CDs are obtained:
- Date supply obtained
- Name and address from whom obtained (e.g. wholesaler, pharmacy)
- Quantity obtained
When CDs are supplied, the regulations require information to be recorded in the CDR, under the following specific headings:
- Date supplied
- Name and address of person or firm supplied
- Details of authority to possess-prescriber or license holder details
- Quantity supplied
- Whether the person who collected the drug was the patient, the patient’s representative or a healthcare professional acting on behalf of the patient;
- If the person who collected the drug was a healthcare professional acting on behalf of the patient, that person’s name and address;
- If the person who collected the drug was the patient or the patient’s representative, whether evidence of identity was requested of that person (yes/no);And whether evidence of identity was provided by the person collecting the drug (yes/no).
These are the minimum fields of information that must be recorded. RPS guidance confirms that additional relevant information can be added without breaking the law.
Electronic Controlled Drug Registers: Pharmacists are allowed legally to keep CDRs electronically (as an alternative to having a bound-book CDs register) if they wish to do so providing they comply with the legislation. The Regulations require that every computerised entry must be attributable and capable of being audited. The computerised register must be accessible from the premises to which it relates and persons authorised by the Secretary of State (e.g., the Society’s inspectors) are able to request that a copy of the register, in its computerised or other specified form, be sent to them.
RPS guidance confirms that CD registers may only be held in a computerised form if safeguards are incorporated into the software to ensure all of the following:
- The author of each entry is identifiable
- Entries cannot be altered at a later date
- A log of all data entered is kept and can be recalled for audit purposes
Access control systems should be in place to minimise the risk of unauthorised or unnecessary access to the data and adequate backups must be made. Pharmacists are also advised that arrangements must be made so that inspectors can examine computerised records during a visit with minimum disruption to the dispensing process. Community Pharmacy England understand that a number of pharmacy system suppliers have now integrated electronic registers into their dispensing systems.
On 1 January 2008, amendments to the Misuse of Drugs Regulations 2001 came into effect in respect of requisitions used for the supply of Schedule 1, 2 and 3 CDs for use in the community. A dedicated requisition form (FP10CDF) (the standard form) was introduced for the supply of Schedule 1, 2 and 3 CDs which should be used unless there were “exceptional circumstances” e.g. where an individual may have difficulty in obtaining the standard form, so a supply could be made in response to an order written on a non-standard form, providing all the legal requirements were met. These changes applied to the supply of Schedule 1, 2 and 3 CDs by authorised practitioners and others. From 30 November 2015, amendments to the Misuse of Drugs Regulations 2001 introduced the mandatory use of the new FP10CDF CD Requisition Form (the mandatory form) for requisitioning all Schedule 2 and 3 CDs. The mandatory use will apply to England, Scotland and Wales; however, Scotland and Wales have their own approved CD requisition forms which professionals in those countries should continue to use. Those who must provide a requisition form to be supplied a Schedule 1, 2 and 3 CD: *Note: exemptions from those who must provide a requisition form include those for use in a prison; or for use in a care home, which as its whole or main purpose provides palliative care for persons resident there who are suffering from a progressive disease in its final stages (e.g. a ‘hospice’). According to the Misuse of Drugs Act 1971 – the primary piece of legislation to the Misuse of Drugs Regulations 2001 – the term “Practitioner” (except in the expression “veterinary practitioner”) means a doctor, dentist, veterinary practitioner or veterinary surgeon. The mandatory form: In Wales, a standard requisition form WP10CDF should be used when ordering a Schedule 2 or 3 CD. Pharmacy contractors in Wales can obtain form WP10CDF from the local Business Service Centre Stores. Marking of requisitions: Submission: Community pharmacies require a private CD account number which should be used when submitting FP10CDF mandatory requisition forms, which is a different account to the NHS account number used by contractors to submit NHS prescriptions. In England, suppliers who need to submit requisition forms but who do not already have a private CD prescription F code must contact their local NHS England team. In Wales, the regulations require that the original completed and signed requisition form WP10CDF be submitted to NHS Wales Shared Services Partnership (NWSSP) – Primary Care Services using the WP34C submission form. Please contact Community Pharmacy Wales (CPW) for further support. Further guidance:
In England, the new mandatory form FP10CDF has been developed to make sure all of the current legal requirements for ordering Schedule 2 and 3 CDs are met. Pharmacy contractors must ensure that requisitions for Schedule 2 and 3 CDs are obtained on the new mandatory form from 30 November 2015. NHS Prescription Services has indicated that requisitions not received on the new mandatory form cannot be processed. The new mandatory form can be obtained online from the NHS Prescription Services website. They can be downloaded, completed online, printed and signed in wet ink.
The regulations also require the supplier of the CD to mark on the requisition their name and address at the time the supply is made. A pharmacy stamp containing the name and address of the pharmacy could meet this requirement.
In England, the regulations require that the original completed and signed mandatory form (FP10CDF) for all Schedule 2 and 3 CDs be submitted to NHS Prescription Services using the submission document FP34PCD* which is available on the NHS Prescription Services website.
General
Q. I have received a prescription for a Schedule 2 Controlled Drug (CD). The date has been computer generated, is this acceptable?
Yes, prescribers can now issue computer-generated prescriptions for all CDs including Schedule 2 and 3 CDs; all details except the signature can be computer-generated.
Q. I have received a prescription for a Schedule 2 CD. The date is missing. Is it acceptable for this to be added by the pharmacist?
No, on prescriptions for Schedule 2 or 3 CDs pharmacists can only amend;
- minor spellings errors; or
- typographical errors; or
- the omission of either words or figures (but not both).
It is not possible for pharmacists to add a missing date or other information such as a missing dose or pharmaceutical form.
Q.Is there a limit to the quantities of a CD that can be ordered on a prescription?
The Department of Health and Social Care has issued strong recommendations that prescriptions for Schedule 2, 3, and 4 CDs are limited to the quantity necessary for up to 30 days’ treatment. Exceptionally, where a prescriber believes that a prescription for a CD should be issued for a longer period, he/she may do so where there is a genuine clinical need, and it does not pose an unacceptable risk to patient safety. Although pharmacists can dispense prescriptions ordering more than 30 days’ supply of any Schedule 2, 3, and 4 CDs, prescribers may be asked to justify their decision for prescribing CDs for an extended duration and should keep a record of their reasons.
Q. Can instalments be ordered on an FP10 prescription form?
No. There is no provision to dispense drugs in instalments on a standard FP10 prescription form or via EPS. The prescriber may not be complying with the General Medical Services (GMS) contract requirements* by not using the form provided specifically for the purpose of supply in instalments. Ultimately, it is for NHS England and Improvement (NHSE&I) to decide whether a general practitioner is compliant with their Terms of Service.
*Part 8 of the National Health Service (General Medical Services Contracts) Regulations 2015 specifies the following prescribing requirements: “Where prescriber orders the drug buprenorphine or diazepam or a drug specified in Part 1 of Schedule 2 to the Misuse of Drugs Regulations 2001 for supply by instalments for treating addiction to any drug specified in that Schedule, he shall—
(a) Use only the prescription form provided especially for the purposes of supply by instalments;
(b) Specify the number of instalments to be dispensed and the interval between each installment; and
(c) Order only such quantity of the drug as will provide treatment for a period not exceeding 14 days.”
Q. Is it a legal requirement to collect a signature on the reverse of FP10/Token?
It is best practice to record the details of the person collecting a Schedule 2 or 3 CD; the reverse of NHS prescription forms and EPS dispensing tokens (FP10DT) have a box for the ‘Signature of a collector of Schedule 2 & 3 CDs’ which can be used to obtain a signature. Any tokens used to collect a signature can be sent to the NHS Business Services Authority (NHSBSA), as appropriate. Alternatively, some contractors may wish to record details of the CD collector electronically (e.g., within the patient’s record). Retaining electronic records within the pharmacy and reducing the use of paper tokens helps to align with the long-term NHS paperless objectives.
FP10MDAs
Q. What drugs are permitted on an FP10MDA form?
FP10MDA forms can only be used for the purpose of ordering a supply by instalments for Schedule 2 CDs, buprenorphine, buprenorphine/naloxone (Suboxone®), and diazepam Single supplies of water for injections can also be prescribed where appropriate, for example when diamorphine dry powder injection is prescribed to be dispensed in instalments.
Q. Is there a limit to the quantity that can be prescribed on an FP10MDA form?
Yes. When using an FP10MDA form, a prescriber must only order a quantity of a CD that provides treatment for a period not exceeding 14 days.
Q. Can I supply a CD outside the directions specified on a FP10MDA prescription without any approved Home Office wording for closure days?
For ‘blue’ FP10MDA prescriptions, the CD should be dispensed in instalments in accordance with the directions given on the prescription. The prescription must be marked with the date of each supply. The instalment direction is a legal requirement and needs to be complied with. However, for certain situations (e.g. if a pharmacy is closed on the day an instalment is due), the Home Office has approved specific wording which provides pharmacists some flexibility for making a supply.
The Home Office approved wording that would cover a closure on a bank holiday is as follows:
“Please dispense instalments due on pharmacy closed days on a prior suitable day.”
FP10MDAs may already include the above wording but, if they do not, the prescription will need to be amended by the prescriber to include the above so that supplies due on a Bank Holiday can be collected on a prior suitable day. Contractors are therefore advised to check their FP10MDA scripts and, if required, get them amended ahead of any closure days for e.g. Bank Holidays. Alternatively, a separate prescription (EPS or FP10) for single supply can be requested to cover the quantity due on the days the pharmacy is closed. Contractors are encouraged to make necessary provisions for dispensing of any prescriptions for controlled drugs with instalments due in advance of pharmacy closure days. If the pharmacy is due to be closed, staff should explain the situation to affected patients and advise them when their dose needs to be collected.
EPS
Q. Can EPS be used to prescribe Controlled Drugs in instalments?
Although CDs may be prescribed using EPS, they cannot be ordered as instalments because there is no equivalent electronic FP10MDA form type available. To order CDs in instalments, prescribers will need to continue using paper hand signed FP10MDA prescription forms.
Q. Do the dispense and claim notification messages for a Schedule 2 or 3 CD prescribed using EPS need to be submitted within 28 days of the date on the prescription?
Legally, all Schedule 2 and 3 CDs must be dispensed within 28 days of the appropriate date. However, for the purposes of claiming reimbursement, pharmacy systems should allow you to send the dispense and claim notification messages after the validity period of 28 days as it is recognised that scenarios may exist (e.g., due to technical/internet outages) where it may not be possible to submit a claim before the 28 days even though medicines were supplied within 28 days.
Your system supplier may alert you to any CD prescriptions yet to be dispensed and approaching their 28-day expiry. Further information is available on Community Pharmacy England’s Period of validity webpage.
Q. On an EPS prescription, can I claim packaged dose (PD) fees for supplying methadone oral liquid in daily dose containers?
Yes, contractors can claim a packaged dose (PD) fee of £0.55 for each separately packaged dose supplied against paper and electronic prescriptions for methadone oral liquid. The PD fee is calculated by adding the number of doses separately packaged minus the number of times the medicine has been dispensed to the patient (patient interactions).
Although there remains no provision to dispense methadone oral liquid in instalments using electronic prescriptions, the PD fee can be claimed for daily dose containers supplied against electronic prescriptions requesting a one-off supply of methadone oral liquid We recommend checking with your system supplier to see if this functionality is enabled on your pharmacy system.
Further information can be found in Community Pharmacy England Briefing: EPS Controlled Drugs (CD) FAQs
Q. Is the quantity that can be prescribed on an FP10MDA prescription limited?
FP10MDA forms can only be used for the purpose of ordering a supply by instalments for Schedule 2 Controlled Drugs plus buprenorphine, buprenorphine/naloxone (Suboxone) and diazepam. The period of treatment is not to exceed 14 days, and the prescriber must specify the number of instalments to be dispensed and the interval between each instalment (Reference: NHS (General Medical Service Contracts) Regulations 2004; Part 3 Regulation 39 (4)).
Q. Can zopiclone 7.5mg tablets be prescribed on an FP10MDA?
Not in England, only Schedule 2 Controlled Drugs plus buprenorphine, buprenorphine/naloxone (Suboxone) and diazepam can be supplied in instalments against an FP10MDA form.
In Wales, Schedule 2, 3, 4 and 5 Controlled Drugs can be supplied in instalments on a WP10MDA form.
Q. Can Suboxone be prescribed on an FP10MDA Prescription?
Suboxone contains buprenorphine and naloxone. The Pricing Authority has confirmed that contractors will be reimbursed for dispensing Suboxone in instalments against Form FP10MDA.
Q. I have received a prescription for Suboxone 2mg tablets, how will this prescription be processed by the Pricing Authority?
Suboxone is available as both 2mg/500mcg and 8mg/2mg preparations. Therefore, a prescription stating a strength of “2mg” alone is not enough to indicate to processing staff which of these strengths has been dispensed, as “2mg” could relate to either of these products.
When a Schedule 2 or 3 Controlled Drug has more than one strength available and the prescribed strength cannot be easily identified from the prescription, it will be returned to you for confirmation of strength to enable correct reimbursement.
If a prescription such this is received, pharmacy contractors should send the prescription back to the prescriber for amendment to the full description of strength to avoid any delay in payment.
Q. Can a prescriber request that a product is dispensed in instalments using an FP10 prescription?
There remains no provision to dispense medicines in instalments on a standard FP10 prescription form. The prescriber may not be complying with the General Medical Services (GMS) contract requirements* by not using the form provided specifically for the purpose of supply in instalments. Ultimately, it is for NHS England to decide whether a general practitioner is compliant with their Terms of Service.
* The National Health Service (General Medical Services Contracts) Regulations 2015 specifies the prescribing requirements:
“Where prescriber orders the drug buprenorphine or diazepam or a drug specified in Part 1 of Schedule 2 to the Misuse of Drugs Regulations 2001 for supply by instalments for treating addiction to any drug specified in that Schedule, he shall—
(a) Use only the prescription form provided specially for the purposes of supply by instalments;
(b) Specify the number of instalments to be dispensed and the interval between each instalment; and
(c) Order only such quantity of the drug as will provide treatment for a period not exceeding 14 days.”
However, payments relating to dispensing additional packaged doses for liquid methadone prescribed on FP10 prescriptions have been introduced – please see the ‘Methadone dispensing (FP10 and FP10MDA)‘ page for further information.
Q. How will I be reimbursed for dispensing in “daily dose containers”? (please note this question is only relevant for CDs eligible to be prescribed on FP10MDAs – excluding oral liquid methadone)
Paragraph 8(15) of the Terms of Service of NHS pharmacists* require that any drug which the pharmacist is required to provide should be in a “suitable container”. Whilst there are no specific provisions that allow prescribers to specify how a drug is supplied, on instructing supply in daily dose containers, the prescriber is not specifying a particular type of container (i.e. glass, plastic, multi-compartment compliance aid, etc.) and so it may be considered that this is not cutting across this Terms of Service requirement where the pharmacist determines what is a suitable container in which to supply the daily doses.
More generally, pharmacists must exercise their professional judgement as to any instruction written on a prescription, having regard to the prescriber’s views. As in all cases, if the pharmacist thinks it appropriate to deviate from the prescription, they should discuss with the patient and/or the prescriber. If a pharmacist does decide to deviate from the prescription, they should make a record of their reasons and if necessary request a new prescription or suggest the prescriber amend their records. Ultimately, it is the pharmacist’s professional obligation to ensure that the patient will be able to take their medicine appropriately and safely; consideration needs to be given to supporting the patients to take the correct daily dose whether this is by packaging in daily dose containers or supplying a suitable measuring device.
*The Terms of Service of NHS pharmacists are set out in Schedule 4 to the NHS (Pharmaceutical and Local Pharmaceutical Services) Regulations 2013
Q. In the bottom left hand side of the FP10MDA form, there is a box to allow pharmacists to record the total number of items on the prescription. Is it mandatory to complete this box?
No, it is not mandatory to complete this box with the total items dispensed but it does serve as a useful check for prescription pricing staff so it is helpful if this box can be completed.
Please note: the number entered in this box should reflect the number of patient interactions/ “pick-ups” from the pharmacy only.
Q. Why is oral liquid methadone subject to different payment considerations to all other drugs which can be supplied on an FP10MDA such as buprenorphine?
The arrangements brought in for oral liquid methadone recognise the difference in workload involved in the dispensing of methadone compared to other drugs on an FP10MDA form. For example, the measurement of the liquid and the entering and maintaining of the controlled drugs register, thus the £2.50 item level fee is only paid against prescriptions for oral liquid methadone.
The “PD” endorsement is only eligible against prescriptions for oral liquid methadone to recognised the workload involved in ensuring the patient is able to measure their medicine appropriately, including interaction with the patient or prescriber to understand whether dispensing in daily packaged doses is appropriate, preparing the daily packaged doses or providing an appropriate measuring device.
For more information on the arrangements for oral liquid methadone prescriptions, please see the ‘Methadone dispensing (FP10 and FP10MDA)‘ page.
Q. Will the changes affect supervised consumption arrangements?
No. Supervised consumption is an enhanced service agreed locally, the funding and subsequently the fees for supervision remain unaffected by these arrangements.
Q. Why were the Payment Arrangements for Oral Liquid Methadone Changed?
Following contractor feedback, Community Pharmacy England reviewed the new methadone payment arrangements introduced in July 2012 by: analysing contractor data taken from a time and motion study which looked at the dispensing costs of supplying methadone, undertaking a methadone prescription audit, and visiting contractors dispensing high volumes of methadone prescriptions for their expert opinions.
The review involved much time and resource in undertaking extensive modelling to fully consider all data received. The new automated audit system that Community Pharmacy England has developed (PRISM) allowed a large prescription study to be carried out which helped to model the costs to contractors of dispensing different methadone prescriptions, i.e. of varying length of treatments and number of patient interactions or “pick-ups.”
The results of the review indicated that on top of the item level fee, paying a separate fee to contractors who package doses of oral liquid methadone into individual containers (either because they have been requested to do so by the prescriber or have chosen to do so for clinical reasons), would be more beneficial than just paying one item level fee.
Q. When can I claim a “Packaged Dose” Fee?
A “Packaged Dose” Fee can be claimed when patients have been provided with separately packaged individual doses in addition to the dose they have come to collect from the pharmacy for that day i.e. either to take at home later on that day or to cover non-collection days.
For example, for a patient collecting three doses on a Friday to cover the weekend:
- If the contractor dispenses all three doses together with a suitable measuring device, the contractor is not eligible to claim for any additional 55p fees.
- If the contractor dispenses the Friday dose as a single dose (e.g. for supervised consumption) with the Saturday and Sunday doses packaged together in one container with a suitable measuring device, the contractor would be eligible for one 55p fee as one separate package was supplied for the Saturday and Sunday doses.
- If the contractor dispenses all three doses in separate containers, the contractor would be eligible for two 55p fees as two separate packages were supplied for the Saturday and the Sunday doses.
If because of the volume, and the size of available containers, one individual dose has to be dispensed between two or more containers, the contractor is only eligible for one (55p) packaged dose fee.
These fees will apply whether or not the prescriber has requested the additional containers on the prescription and will apply to both FP10 and FP10MDA prescriptions for oral liquid methadone.
Q. How will the 10p payment for containers be paid for on an FP10MDA prescription for oral liquid methadone?
The 10p payment for containers is paid to contractors on those occasions where the prescribed quantity requires a contractor to split a pack in order to supply the exact quantity as the quantity prescribed is not available in a manufacturer’s original pack/listed pack size in the Drug Tariff.
Where oral liquid methadone is prescribed, this 10p payment will only be made where the total quantity prescribed (sum of all the doses) is outside of a pack size listed in the Drug Tariff (or a multiple of a listed pack size) as the new payments include a contribution to the cost of the container supplied.
For all other drugs prescribed on an FP10MDA form, the 10p payment will be made at each patient interaction or ‘pick-up’ from the pharmacy, regardless of whether the quantity prescribed per interaction is available as listed pack size.
Q. In the bottom left hand side of the FP10MDA form, there is a box to allow pharmacists to record the total number of instalments provided (i.e.pick-ups) on the prescription. Is it mandatory to complete this box?
No, it is not mandatory to complete this box with the total number of instalments dispensed but it does serve as a useful check for prescription pricing staff so it is helpful if this box can be completed.
Please note: the number entered in this box should reflect the number of instalments supplied or “pick-ups” from the pharmacy only and not any additional packaged doses (those are paid according to the separate endorsement).
Q. How will I be reimbursed for dispensing oral liquid methadone in “daily dose containers”?
The £2.50 item level fee and, where endorsed, additional packaged dose fees payable against prescriptions written for oral liquid methadone is to help support the contractor with the costs of the additional workload involved in ensuring the patient is able to measure their medicine appropriately, including interaction with the patient or prescriber to understand whether dispensing in daily packaged doses is appropriate, preparing the daily packaged doses or providing an appropriate measuring device.
The additional packaged doses for methadone must be endorsed clearly to ensure payment. Please see above for endorsement requirements.
Q. Do I have to supply in daily dose containers where the doctor has instructed this on a prescription, and what if the doctor has not specified daily dose containers?
Paragraph 8 (11) of schedule 4 of the 2013 regulations (The Terms of Service) requires the pharmacist to first decide whether it would be most appropriate to provide oral liquid methadone as:
(a) Each dose in a separate container;
(b) An original pack (or original packs); or
(c) The oral liquid methadone in some other way.
This may require that the pharmacist discuss with the prescriber and/or the patient, the most appropriate way of supplying oral liquid methadone to the patient concerned. For some patients, and with some dosages, a suitable measuring device will be sufficient, allowing the supply of a single container to last for several days. However, in other cases, the patient’s safety and accurate measurement of the oral liquid methadone will require the pharmacist to dispense individual doses in separate packages.
Where a prescriber has specified that each dose of the medicine should be packaged separately, the contractor must exercise their professional judgement having regard to the prescriber’s views.
The absence of any request on the prescription for daily dose containers should not be taken as an indication that the supply in a bulk container is appropriate – that assessment must be made by the pharmacist.
Once the pharmacist has decided upon how best to supply the medicine, they must package in accordance with their decision and endorse the prescription appropriately for any additional separately packaged doses as outlined in Part IIIA of the Drug Tariff.
They may also wish to keep a record e.g. on PMR of the assessment carried out and decisions made in case of future enquiries.
Q. Are the item level fee and “PD” endorsement applicable to all controlled drugs which can be supplied on an FP10MDA form (e.g. buprenorphine)?
Payment for other controlled drugs which may be prescribed on an FP10MDA form (e.g. diazepam or buprenorphine) is based on the number of patient interactions or “pick-ups” from the pharmacy only. No item level fee is applied; nor can the fee for additional packaged doses be claimed.
Q. Why are these fees only for oral liquid methadone and not for all other drugs which can be supplied on an FP10MDA such as buprenorphine?
The arrangements recognise the difference in workload involved in dispensing oral liquid methadone compared to other drugs on an FP10MDA form; thus the £2.50 item level fee and, where endorsed, 55p for each additional packaged dose, are only paid against prescriptions for oral liquid methadone.
Q. Will the changes affect supervised consumption arrangements?
No. Supervised consumption is an enhanced service agreed locally, the funding and subsequently the fees for supervision remain unaffected by these arrangements.
Q. Can a prescriber request that a product is dispensed in instalments using an FP10 prescription?
There remains no provision to dispense medicines in instalments on a standard FP10 prescription form. The prescriber is not complying with the GMS contract requirements* by not using the form provided specifically for the purpose of supply in instalments.
* The National Health Service (General Medical Services Contracts) Regulations 2015 Regulation 56, paragraph 10(a) – (c) specifies the prescribing requirements:
“Where a prescriber orders the drug buprenorphine or diazepam or a drug specified in Part 1 of Schedule 2 to the Misuse of Drugs Regulations 2001 for supply by instalments for treating addiction to any drug specified in that Schedule, he shall—
(a) Use only the prescription form provided specially for the purposes of supply by instalments;
(b) Specify the number of instalments to be dispensed and the interval between each instalment; and
(c) Order only such quantity of the drug as will provide treatment for a period not exceeding 14 days.”
However, please see the endorsement guidance above for payments relating to dispensing additional packaged doses for liquid methadone prescribed on FP10 prescriptions where only one patient interaction occurs (e.g. a weekly supply but the patient only comes into the pharmacy to pick-up on the Monday).
Related resources
Download our Factsheet: Dispensing prescriptions for Controlled Drugs
Download our Factsheet: Endorsing instalment forms correctly
The Misuse of Drugs Regulations 2001 (16(2)) (external)
List of electronic CD register suppliers
NICE guidelines- “Controlled drugs: safe use and management” (April 2016)
NHS guidance: The Controlled Drugs (Supervision of Management and Use) Regulations 2013 (November 2013)
Department of Health: Best Practice Guidance – Controlled Drugs (Supervision of Management and use) Regulations 2013 (February 2013)