NHS medicines database (dm+d)
Published on: 17th July 2013 | Updated on: 21st August 2023
 The NHS Dictionary of Medicines and Devices (dm+d) is the NHS standard dictionary for medicines licensed in the UK.
The NHS Dictionary of Medicines and Devices (dm+d) is the NHS standard dictionary for medicines licensed in the UK.
The use of ‘standards’ such as dm+d within the health sector enables people, devices and systems to communicate in a more efficient and electronic manner. It is important that those that can such as manufacturers and wholesalers keep dm+d accurate by updating it when there are changes.
Electronic systems that exchange or share information about medicines relating directly to a patient’s care must adhere to this standard by using dm+d identifiers and descriptions when transferring information.
See also our dm+d and EPS summary one-pager factsheet or dm+ briefing with images of dm+d content.
dm+d browsers: Pharmacy team members have several options (see external links at the bottom of this page) and the section below,
dm+d is a cornerstone of the Electronic Prescription Service. dm+d provides a unique code for each medicine and device along with a textual description of the item and as both pharmacy and prescribing systems have adopted dm+d, it supports interoperability by allowing these diverse clinical systems to ‘talk the same language’.
Pharmacy and GP system suppliers adopt or map to the information included within it. Each medicinal item has an entry in the database in which information is stored in several interlocking levels; there is information relevant to pharmacy
teams in each level.
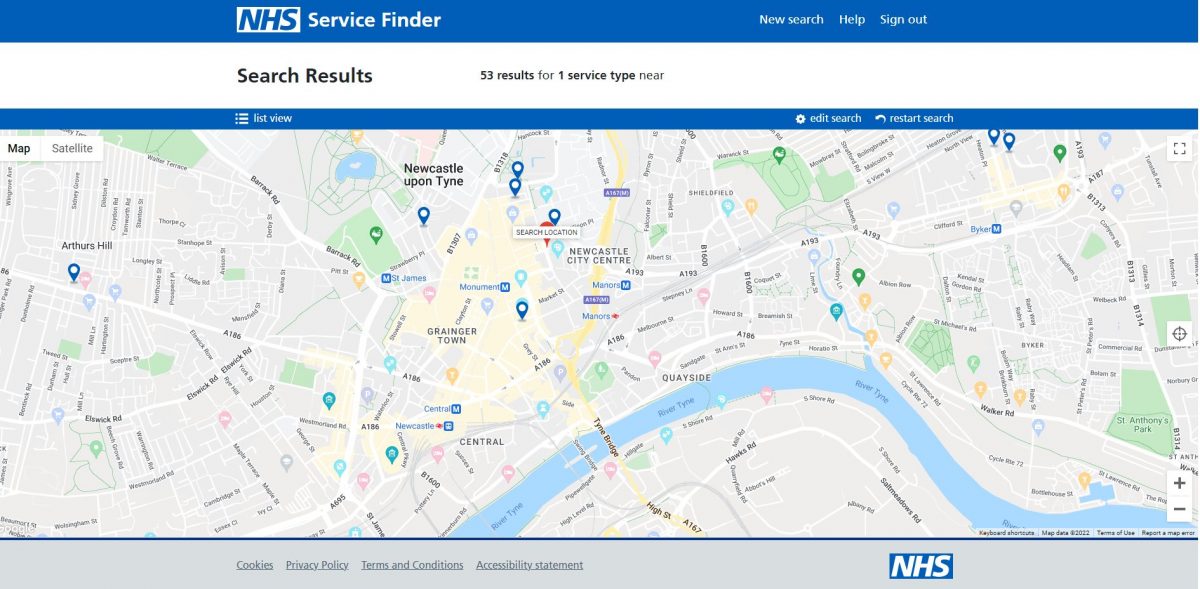
List of dm+d browsers
Key information held in the dm+d can be accessed online through dm+d viewers.
Several options are listed at the bottom of this webpage and also below
| dm+d browser options | How to access and notes. |
| dm+d browser (OpenPrescribing). Information about this newer viewer can be read here. | Freely usable (see link on left). |
| NHSBSA dm+d browser. | Freely usable (see link on left). |
| Unilexon dm+d browser | Freely usable (see link on left). |
| East Kent NHS dm+d browser | Freely usable (see link on left). |
| Additionally there is a Datapharm dm+d browser at Medicines.org.uk (login required, and may be created on the basis of your NHSmail) | Link on left. Login required, and may be created on the basis of your NHSmail. |
How to use dm+d viewers?
The dm+d can be a useful source of information to pharmacy team members providing information on a product’s attributes, for example whether a particular product is reimbursable, whether broken bulk can be claimed or whether the product is recognised as a special container or calendar pack. However, it is important to note though that as different systems suppliers have implemented dm+d in different ways, the online viewers may contain more information that is available through pharmacy systems.
As the reimbursement rules can be impacted by the way a particular dispensed product has been prescribed or other items on the prescription form, care has to be taken in interpreting the information on dm+d. Common queries relate to:
Reimbursement price of the product
dm+d provides indicative prices for actual products. If a product is in Part VIII and prescribed generically, reimbursement is based on the Part VIII price and not the list price of the actual product dispensed.
Indicative nature of the prices
Prices on dm+d are extracted from the NHSRxS database, however as prices in the market change the indicative price may differ from the actual price used to calculate reimbursement. For example where the price of a generic medicine (other than Part VIII Category M) changes before the 8th of the month, the reimbursement price will be changed with effect the 1st of that month. It is also important to keep in mind that there may be a time lag between a price changing, dm+d being updated and the latest release of dm+d then being added to pharmacy systems.
Schedule 1 products (drugs, medicines and other substances that may not be ordered under the NHS)
Subject to the prescriber having the appropriate prescribing rights, any food, drug, toiletry or cosmetic may be prescribed on an NHS prescription unless the product is listed in the schedule 1 to the NHS (General Medical services Contracts) (Prescription of Drugs etc.) Regulations 2004 which is reproduced in Part XVIIIA of the Drug Tariff). As an exception to this rule, Schedule 1 products can be dispensed where a product is prescribed generically and the generic product is not listed in schedule 1 and the name of the product has a recognised ‘official title’. Therefore it may still be possible to dispense certain actual products where the schedule 1 field in dm+d is marked ‘yes’.
NHS Business Services Authority (NHSBSA) is collecting feedback before the full launch of its new NHSBSA Dictionary of medicines and devices (dm+d) browser. Feedback from pharmacy teams and other users will help NHSBSA to improve their dm+d browser.
The newer NHSBSA dm+d browser was launched in 2022 superceding the legacy NHSBSA dmd browser.
Pharmacy teams will still have access to the other free dm+d browsers:
What are the features of NHSBSA’s dm+d browser?
All dm+d browsers enable searching of products by generic (virtual medicinal product, VMP) or brand (actual medicinal product, AMP) name, or by supplier name. The new browser includes the following updated features:
- enhanced search functionality by product codes
- display of product barcodes
- compatibility with different devices including smartphones; and
- additional invalid and discontinued product information.
Survey opportunities
The NHS England Pharmacy Terminology team are welcoming feedback to help shape the direction of dm+d usage and structure:
|
VMP Drug Forms in dm+d – supplier thoughts and views are needed please The NHS England Pharmacy Terminology team is keen to understand if you use the VMP <Drug_Form> attribute within dm+d. We are undertaking a piece of discovery work as part of UK Medicines Terminology Futures. Please help by responding to this very short survey. The survey should only take a few minutes to complete and your feedback is invaluable to us so we would be very grateful if you are able to respond. Please be assured that any personal details you submit will be treated in strict confidence. Thank you in advance and if you have any questions about completing this survey, please contact: nhsdigital.ukmeds@nhs.net. For further information about this change, please visit: Updating of NHS dm+d ‘Forms’ at virtual medicinal product level – NHS Digital |
Feeding back about your use of dm+d browsers
If you’ve tested the NHSBSA dm+d browser and wish to suggest further improvements please email NHSBSA at: nhsbsa.cdrsupport@nhs.net.
Feed back about the OpenPrescribing dm+d viewer can be sent to ebmdatalab@phc.ox.ac.uk.
Community Pharmacy England also shares feedback to NHSBSA and OpenPrescribing about their browsers and future developments.
Community Pharmacy England has liaised with the dm+d Editorial Board on a number of issues relating to the Dictionary’s editorial policy and how particular products are represented in the Dictionary Structure. Community Pharmacy England is monitoring the implementation of the Dictionary and will continue to raise issues as necessary with NHS Digital, the Pricing Authority or others.
Community Pharmacy England may also liaise with those that manage dm+d viewers.
This section sets out technical information about dm+d for advanced users within community pharmacy teams
Data structure
The dictionary is structured around four key components: Virtual Medicinal Product (VMP), Actual Medicinal Product (AMP), Virtual Medicinal Product Pack (VMPP) and Actual Medicinal Product Pack (AMPP).
The Virtual Medicinal Product (VMP) describes the generic title for a product including the form and strength, for example ‘Atenolol 100mg tablets’.
The Virtual Medicinal Product Pack (VMPP) describes the generic title for a generic or proprietary product pack which is known to have been available. The description includes the pack size, for example ‘Atenolol 100mg tablets 28 tablet’.
The Actual Medicinal Product (AMP) describes an actual product which is known to have been available linked to the name of a particular supplier, for example ‘Tenormin 100mg tablets (AstraZeneca UK Ltd)’.
The Actual Medicinal Product Pack (AMPP) describes an actual product which is known to have been available linked to both the name of a particular supplier and information on the pack size of the product, for example ‘Tenormin 100mg tablets (AstraZeneca UK Ltd) 28 tablet 2 x 14 tablets’.
Linked to each component, the dictionary also includes information to support the prescribing and dispensing process, for example linked to the ‘Actual Medicinal Product Pack’ component there are a range of attributes detailed such as the product’s legal category and confirmation of whether a product is considered a special container or calendar pack. This information is populated by the Pricing Authority.
Coverage of dm+d
It is estimated that dm+d contains over 99.9% of medicines and appliances prescribed in primary care. Items not included in dm+d include certain extemporaneously dispensed and special formulation products. If a GP wishes to prescribe a medication item which is not listed in the dm+d, an electronic prescription cannot be issued by EPS and current paper prescription processes should be followed.
NHSBSA work to ensure licensed products are on dm+d by product launch date, however it may still take time for an individual system supplier to update their systems with the latest release of dm+d; typically, suppliers update their systems monthly.
System changes that pharmacy staff may notice
Depending on what functionality has been available historically in a particular system, where dm+d has been adopted, as the information in dm+d is sourced from the NHSRxS database, pharmacy staff may notice improvements in the accuracy of dispensing and endorsing guidance provided by the systems. For example information on whether a product can be prescribed on an NHS prescription. Different suppliers are likely to make use of this information in different ways.
Other changes that staff may notice:
Word order: Staff may also notice a change in the description of products in their systems with products listed in dm+d described according to the dm+d editorial policy. For example, dm+d describes products in the order: product, strength, formulation. Some pharmacy systems have traditionally described products in the order: product, form, strength.
Word conventions: In some cases dm+d may use different words to those found historically in prescribing and dispensing systems. For example ‘enteric coated’ tablets are described as ‘gastro-resistant’, and for inhalers, dm+d uses the term ‘dose’ as opposed to ‘actuation’, ‘inhalation’ or ‘puff’.
Terminology length and abbreviations: In some cases, the descriptions defined by dm+d are much longer than the titles used in existing supplier drug databases, this could mean space pressure on paper outputs. In some cases, it will not be possible to print a product’s full name on a dispensing label because of space restrictions; in this case, the system may automatically abbreviate the name of the product to fit on the label. For many products, dm+d includes both the full name and an abbreviated name for this purpose.
Pick lists: As well as noticing subtle changes in the description of products, pharmacy staff may also notice that the location of products on ‘picking lists’ in systems changes depending on how a supplier has implemented dm+d within their system. Also in some systems, the description of a product on-screen may be subtly different from the description used in the picking list, for example where a supplier has chosen to abbreviate information in picking lists.
Quantities and units of measure: For inhalers, dm+d expresses the number of doses contained within each inhaler, for example rather than the prescription requesting ‘1 inhaler’, the prescription may read ‘200 dose’.
dm+d entry changes
dm+d’s editorial policies have set out standards for changes to dm+d entries for example setting out that
- “the Actual Medicinal Product name may change over time”; and
- “if the name of an AMP changes the dictionary maintainer will ensure a history of the change is maintained.”
The position of Community Pharmacy England and Community Pharmacy IT Group is that is important that those that can such as manufacturers and wholesalers keep dm+d accurate by updating it when there are changes.
Automated calculation of reimbursement at the Pricing Authority
When a prescription is priced by the Pricing Authority, payment is based on the prescribed product and the prescription is checked for any pharmacy endorsements where further information is needed such as pack size information or information on the product supplied if the product is not in Part VIII of the Drug Tariff.
As EPS Release 2 is introduced, payment of electronic prescriptions will be based on the product, sent as a dm+d code, listed in the product field of the message. Where additional information is required to price the prescription, this will need to continue being provided by the pharmacy but through endorsements in the electronic message sent to the Pricing Authority rather than hand endorsements on a paper form.
A problem at present relates to attempts to add product information so that it appears to the pharmacy team within the dose area (the incorrect place). Read more at: cpe.org.uk/dosageissue.
Additional info for system suppliers and advanced users
dm+d information such as databases are also accessible in database format for those registered at NHS Digital TRUD.
Read more at: NHSBSA’s dm+d webpage.
The position of Community Pharmacy England and Community Pharmacy IT Group is that is important that those that can such as manufacturers and wholesalers keep dm+d accurate by updating it when there are changes.
dm+d accuracy and accuracy of related systems helps the NHS to accurately record its costs. It can help pharmacies to be fairly reimbursed e.g. contractors are reimbursed at dm+d listed price for non-Part VIII (Drug Tariff) items. dm+d data quality also help the clinical advice and patient outcomes.
It is strongly recommended that manufacturers and wholesalers and other dm+d administrators help to keep dm+d up-to-date and accurate.
Contractors may also query manufacturers which do not keep their prices up-to-date.
See some other comments about dm+d and entry changes within the ‘Technical info’ of this webpage.
System supplier may map their drug databases to dm+d, and some must map to dm+d e.g. EPS prescribing / dispensing systems which enable EPS.
Mapping process: overview
Prescribers can issue EPS prescriptions using either the generic VMP or the supplier-linked AMP code. Most GP and pharmacy system suppliers use drug databases in their systems, and have to ‘map’ the dm+d products to products listed in their databases so that EPS messages can be sent and received.
Mapping timing/frequency: EPS R2 prescribing / dispensing system suppliers must all perform a mapping exercise to synch to many dm+d listings, at least once every two months. Sometimes GP practice or pharmacy staff must update their systems to sync with the most recent mapping. All pharmacy system suppliers map at least monthly or weekly. Community Pharmacy England’s position is that more frequent mapping is critical for taking into account Drug tariff and dm+d changes in a timely manner (e.g. new/changed items etc.). Prompt synchronizations and updates to systems around the start of the month are particularly important because those can incorporate newer Drug Tariff changes.
NHSBSA release dm+d tables for suppliers every week so suppliers can frequently map. System suppliers synchronizing frequently supports with pharmacy teams being fairly reimbursed e.g. by enabling prompts that the reduce the risk a non-allowed appliance or item could be dispensed inadvertently if the prompt is noticed.
EPS users should ensure their systems are up-to-date because non-updated systems may not incorporate the newest data available e.g. lists of non-allowed items.
Mapping of system supplier drug databases to dm+d
A prescriber will issue a prescription for a product via the EPS service using either the VMP or AMP code.
System suppliers currently use a variety of drug databases in their systems, in some cases these are maintained in-house, in other cases a supplier may purchase their database from an external company. Where a supplier has not adopted the NHS dm+d as their core database, they must ‘map’ the codes of the individual products on their database with the dm+d to send messages via the electronic Prescription service. Electronic messages will contain the dm+d codes and descriptions even where a supplier has adopted a mapped solution.
An issue that may arise is that if an individual GP system supplier has not mapped a particular item held on their drug database with the dm+d code, that GP will not be able to prescribe that item electronically. The current requirement on GP system suppliers to undertake at least 95% mapping of their drug databases to dm+d based on the most frequently prescribed drugs in primary care. This equates to just over 2,100 products. The dm+d programme will continue to work with system and drug database suppliers to ensure the level of mapping increases and is maintained when new products become available in England.
Community Pharmacy England’s position is that prescribing system suppliers should continue to map to greater numbers of dm+d listings so that more prescriptions can be sent via EPS instead of via paper prescriptions.
Reporting medicine ‘mapping errors’ and (EPS)
Where a particular item held on the GP system’s drug database is not mapped with a dm+d code, or the mapping is inaccurate, there may be risk of medicolegal consequences. If pharmacy teams spot that information printed on the token is different from prescribing information shown on the screen, they should report it to their pharmacy system supplier immediately and also report it as a patient safety incident using the Learn from patient safety events (LFPSE) service process.
If there is a listing which is present on dm+d but not listed within the pharmacy system (e.g. preventing an AMPP being selected as dispensed where you wish to do so), then contact your pharmacy system supplier.
Prescribers may also contact their prescribing system to request that additional items on dm+d are mapped, so that more can be prescribed via EPS.
For other types of reporting note the section below.
For information about reporting dm+d system mapping errors, please refer to the ‘Mapping of systems to dm+d’ section of this webpage and the ‘Reporting sub-section’ (just above).
For other types of reporting, note that there are also dm+d reporting options at NHSBSA’s dm+d webpage.
The dm+d is providing a standard description and electronic identifier for all medicines but at present there is no standard structure to represent dosage instructions integrated with dm+d and pharmacy systems e.g. “Take two tablets three times a day.” Read more at Computable dose instruction standards.
Further info & dm+d browser links
dm+d browser options
- OpenPrescribing dm+d viewer (free) (read more about this viewer)
- NHSBSA dm+d browser
- Other viewers: Unilexon; East Kent NHS; Datapharm (login & registration required)
Read more and watch webinars:
- NHSBSA’s dm+d webpage including webinars
- Community Pharmacy England: dm+d and EPS summary factsheet and dm+d briefing.
Further info:
- If you have IT queries on this webpage or you require more information about this webpage please contact it@cpe.org.uk. For dispensing/pricing queries (e.g. relating to specific dm+d items) contact Dispensing & Supply Team. To share and hear views about digital developments with like-minded pharmacy team members, join the CP Digital email group today.
See also: Pharmacy reimbursement
Return to the Pharmacy IT hub ; Standards and Interoperability IT a-z index