Prescription returns
Published on: 17th July 2013 | Updated on: 23rd January 2024
See our Community Pharmacy England Briefing 020/22: Understanding prescription returns and disallowed items.
Where the NHS Business Services Authority (NHSBSA) cannot process a prescription item for payment due to insufficient endorsed information, it is returned to the pharmacy for the missing information to be added so that it can be correctly reimbursed. From 1 February 2024 all prescriptions that have been referred back or disallowed must be completed and returned to the NHSBSA no later than 18 months from the date they are first sent to the pharmacy.
Part II Clause 9F of the Drug Tariff states ‘where insufficient information is available to enable the Pricing Authority to process the prescription, including where it was submitted for a SSP payment, the form (or a copy of the original form) shall be returned to the contractor who shall endorse the prescription form (or copy) with the information requested. Returned prescriptions shall be priced using the Drug Tariff relevant to the month in which the prescription form (or copy) is returned to the Pricing Authority.’
In 2021, just under 800,000 items were referred back (RB) to pharmacy contractors by the NHSBSA for further clarification. This works out to a monthly average of 66,458 items (0.07% of all prescriptions items processed) or ~6 items per pharmacy contractor per month.
| Year | Items processed | Total items referred back |
| 2021 | 1.139bn | 798k |
The most common reason why an item is returned is due to an incomplete endorsement, where one or more of the following is missing:
- manufacturer / brand name (if not in Drug Tariff and listed by more than one supplier)
- pack size (if more than one pack size is available)
- price (if NHSBSA does not hold a price for it)
- formulation (commonly seen with handwritten prescriptions for products which are available in multiple presentations).
An item could also be returned if too much information is included in the endorsement, for example more than one manufacturer endorsed.
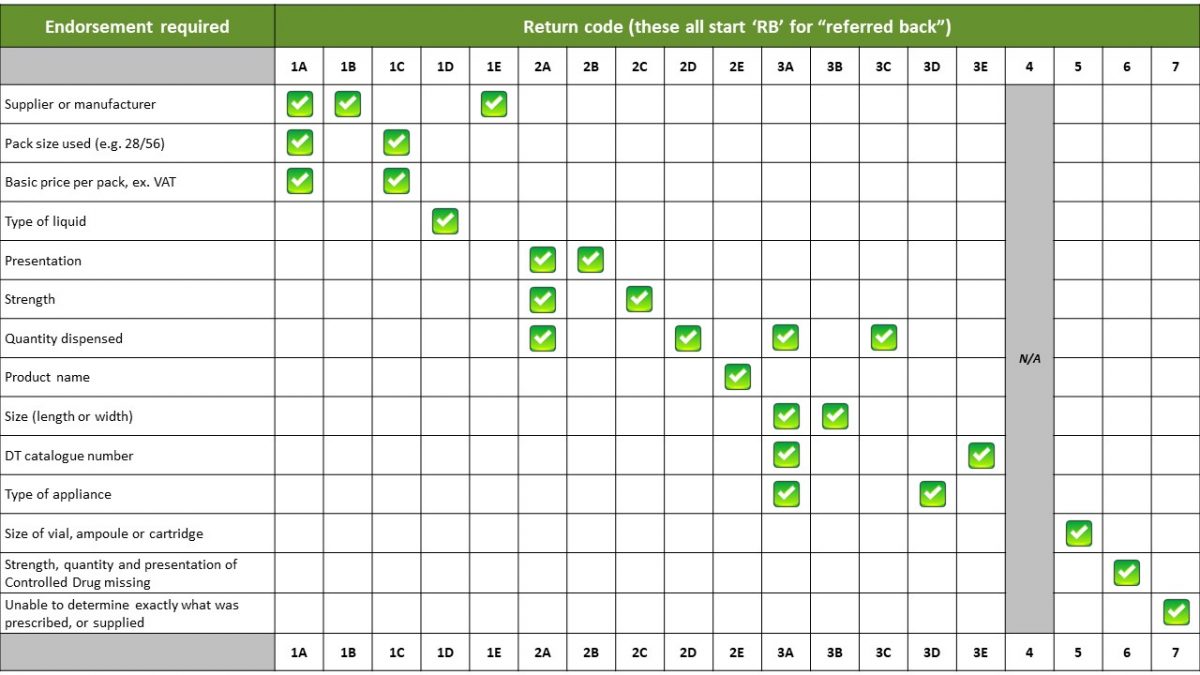
The NHSBSA apply a coding system to identify up to 19 possible reasons for items being referred back to the pharmacy for further information. Each returned item is assigned a referred back code to indicate the referred back reason.
| Missing or incorrect patient exempt or paid declarations are not referred back to contractors – if patient declarations are missing this can lead to switching and potentially inappropriate charge deductions. |
Returns code table
The following table explains what each return code means:
From July 2022 (for the dispensing month of June 2022), all new prescription returns/referred back items and disallowed items will be received through the MYS portal and MYS will be the only route available to view and submit required information for all these items.
NHSBSA sends out a notification email to the pharmacy NHSmail account if any new referred back or disallowed items have been generated for the contractor to complete on their MYS account. Contractors can view any referred back items for completion by checking the ‘Unpaid items’ tab on MYS landing page. Using MYS for receiving digital referred back and disallowed items allows contractor to:
- receive these items sooner and removes the risk of paper returns getting lost in transit;
- provide information required to processes referred back items faster;
- submit a challenge for any disallowed items electronically;
- track the progress of any referred back items throughout until the point they are processed;
- generate monthly reports showing the status of any referred back items.
When the required additional information has been added to the relevant sections within MYS, contractors can submit referred back items electronically to the NHSBSA for processing.
It is important to note that prescription returns via MYS are only held in the system for a period of 18 months from the date they are first sent to the pharmacy for action; if contractors have not completed and returned any outstanding referred backs before this deadline has passed, the referred back items will be deleted from system. Contractors already using MYS for referred backs should check if any items are awaiting completion before they expire.
During the end of month submission process, the total number of completed RB prescription forms and items being resubmitted should be declared in the relevant boxes in of the digital MYS submission form.
Please note only the number of items returned for clarification where payment has been delayed should be declared on the digital MYS submission form. This is because payment will already have been received for any other items on the returned forms.
On the digital MYS submission form:
- Include the number of prescriptions being resubmitted in with your end of month figures in the appropriate exempt or paid box.
- Include the number of items being resubmitted in with your end of month figures in the appropriate exempt or paid box.
- Ensure that figures for any resubmitted prescriptions/items charged at the old charge rate are declared with your end of month figures.
- Add the total number of items and prescriptions being resubmitted to your end of month prescription totals.
Referred back items submitted for payment are priced using the Drug Tariff relevant to the dispensing month in which the prescription return is received by the NHSBSA. Delays in the completion of missing information for referred back can affect the timing of payments. This can create potential cash-flow risks for contractors if very expensive items are referred back, or a large number of items are returned to the pharmacy to complete the missing information. If the submitted information is still insufficient to allow NHSBSA to process the prescription for payment, the item will be re-sent to the contractor.
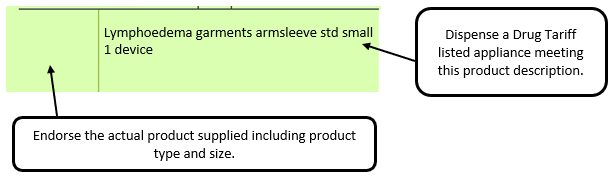
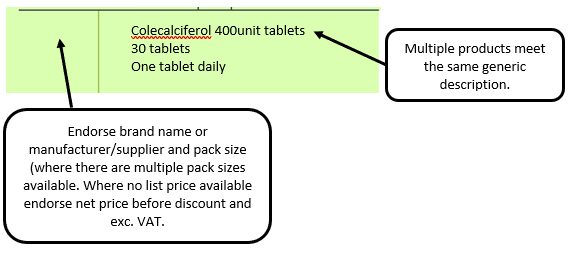
Example 1 – Supplier or manufacturer details missing – appliance
e.g. where a generic prescription is received for an appliance and multiple products meeting that description are listed in the appliances section of the Drug Tariff
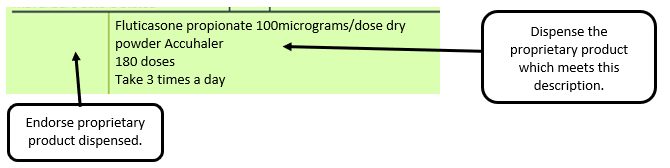
Example 2 – Product name missing
e.g. where a generic prescription for an inhaler includes a trademarked name
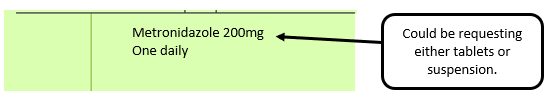
Example 3 – No pharmaceutical form listed on the prescription
Action: Return to the prescriber to be amended to include pharmaceutical form.
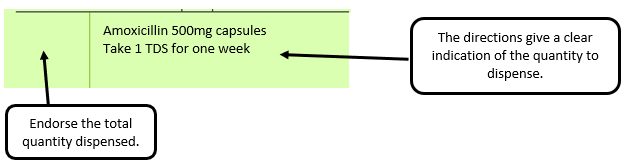
Example 4 – Total quantity not stated but clear from dosage instructions
Example 5 – Endorsement of non-Part VIIIA generic products
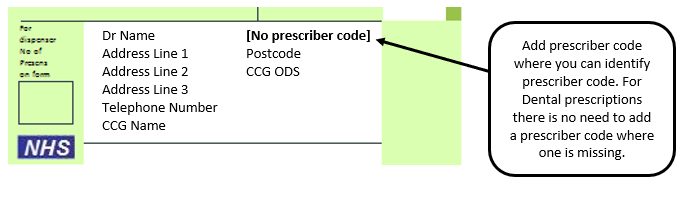
Example 6 – Missing prescriber code
Please note: Where a contractor provides the drugs or appliances ordered on any NHS prescription form, except forms issued by a Dental Practitioner, the Pricing Authority may return the prescription form to the contractor (as a referred back) if there is no prescriber code(s) present on the prescription form and request that the contractor seek the appropriate code(s).
A disallowed item is one that has NOT been passed for payment by the NHSBSA. Disallowed items should not be confused with items which are referred back to contractors for further clarification because insufficient information was available for the NHSBSA to process the prescription(s) for payment (for example, due to missing endorsements).
From July 2022 disallowed items will be received through the MYS portal and MYS will be the only route available to view and submit required information for all these items. Disallowed items via MYS will include the item number and disallowed reason via the MYS portal. Disallowed items via MYS can arrive daily and the NHSBSA will send out a notification email to the pharmacy NHSmail account if any new disallowed items have been generated.
If it is believed that an item has been incorrectly disallowed by the NHSBSA, contractors may submit a challenge to the NHSBSA, who will investigate the issue and rectify any missing payments if a processing error is identified.
To challenge disallowed items returned via MYS, contractors can click on a link available under the disallowed item displayed on the portal. A free-text box is provided for a contractor to provide a reason as to why they believe the item has been disallowed in error, and to provide a contact email address. Once a challenge is successfully submitted, the information is sent directly to the NHSBSA Helpdesk to action, removing the need for contractors to send a separate email or make a telephone call.
The outcomes of a challenge from an MYS perspective would either be for the item to remain as a disallowed item if it was deemed that the item was correctly disallowed, or the status of the item would be changed in MYS from a disallowed item to a referred back item to allow the contractor to provide any additional information required by the NHSBSA to process the item for payment.
If a pharmacy contractor remains unsatisfied with outcome of a disallowed item challenge, they can contact Community Pharmacy England’s Dispensing & Supply team on 0203 1220 810 or email their query to info@cpe.org.uk for further advice on the matter.
Community Pharmacy England closely monitors problems with copies of prescriptions being returned for clarification incorrectly. Please report suspected incorrect returns to the Community Pharmacy England Dispensing and Supply Team who will ensure that this is fed back to the Pricing Authority.
Community Pharmacy England Top Tips
|
The NHSBSA Prescription Services helpline can be contacted for further advice if pharmacy staff are unsure as to why an item has been returned or if it is unclear as to what needs to be endorsed. Telephone: 0300 330 1349 or 0191 279 0568; email: nhsbsa.prescriptionservices@nhsbsa.nhs.uk
Q. Can I receive reports of items referred back to the pharmacy each month?
A. Contractors view reports on the MYS portal. The portal shows how many RBs were received from the NHSBSA, how many have been re-submitted, how many are awaiting action by the NHSBSA and how many have been successfully processed. The reports help to keep track of RBs and can be used as a learning tool for pharmacy staff to help reduce the number of RBs by endorsing prescriptions correctly.
Contractors can also check whether an item was referred back by checking their monthly Prescription Item reports (PIRs). The product will be marked in column AP (column heading ‘RB’) if an item has been RB. Column AQ (column heading ‘RB value’) displays the RB code for which the item was RB. To access item reports, contractors should register for the Information Services Portal on the NHSBSA’s website.
Q. Do completed RB items need to be declared on my digital MYS submission form?
A. Yes, the total number of completed RB prescription forms and items being resubmitted should be declared in the relevant boxes on the digital MYS submission form. Please note only the number of items returned for clarification where payment has been delayed should be declared on the digital MYS submission form. This is because payment will already have been received for any other items on the returned forms.
Related Resources
Using Your Schedule of Payment to Monitor Performance
Community Pharmacy England Briefing 020/22: Understanding prescription returns and disallowed items