Price Concessions
Published on: 28th February 2020 | Updated on: 26th April 2024
What is a price concession?
When community pharmacies cannot source a drug at or below the reimbursement price as set out in the Drug Tariff, the Department of Health and Social Care (DHSC) can introduce a price concession at the request of Community Pharmacy England. A price concession can be requested for any drugs listed in Part VIIIA, Part VIIIB and Part VIIID of the Drug Tariff. For any drugs granted price concessions, contractors are automatically reimbursed at the new prices for that month.
The Department of Health and Social Care (DHSC) has today (26/04/2024) confirmed the following initial list of price concessions for April 2024. The individual updates can be found here: No additional endorsements are required for price concessions. A price concession only applies for the month it is granted; any prices agreed for concessions requested late in the month will roll over into the following month, unless Community Pharmacy England requested it late in the month and it has been agreed the price will roll over into the following month. Rolled over prices will be identified as such. We encourage pharmacies to report any problems obtaining a Part VIII product at or below the stated Drug Tariff price, using the online feedback form on the our website. Please include full details of the supplier and price paid for any products sourced above the Drug Tariff price. We will investigate the extent of the problem and, if appropriate, discuss the issue with DHSC. We are still working with the DHSC to agree concessionary prices for other drugs reported to be unavailable at the stated November 2023 Drug Tariff price. Please note that we cannot provide details of any generic products awaiting price concession approval from DHSC. Contractors will be alerted to further updates to the price concession list through our website and via our e-news email. If you wish to subscribe to our email list, you can receive an email as soon as any announcements are made.
Drug
Pack size
Price concession
Amoxicillin 250mg/5ml oral suspension
100
£2.16
Anastrozole 1mg tablets
28
£1.38
Aripiprazole 10mg tablets
28
£6.88
Aripiprazole 15mg tablets
28
£7.30
Aripiprazole 5mg tablets
28
£6.12
Azithromycin 250mg capsules
6
£4.45
Baclofen 10mg tablets
84
£2.71
Bimatoprost 100micrograms/ml eye drops
3
£4.33
Bimatoprost 300micrograms/ml / Timolol 5mg/ml eye drops
3
£8.75
Bumetanide 1mg tablets
28
£2.46
Calcipotriol 0.005% / Betamethasone dipropionate 0.05% gel
60
£22.91
Calcipotriol 0.005% / Betamethasone dipropionate 0.05% ointment
30
£10.11
Cefalexin 250mg capsules
28
£2.04
Cefalexin 500mg capsules
21
£2.46
Chloramphenicol 0.5% eye drops
10
£4.35
Cinacalcet 30mg tablets
28
£7.79
Clarithromycin 125mg/5ml oral suspension
70
£4.03
Clarithromycin 250mg/5ml oral suspension
70
£5.50
Co-amoxiclav 250mg/125mg tablets
21
£2.44
Co-amoxiclav 500mg/125mg tablets
21
£3.84
Co-careldopa 25mg/100mg tablets
100
£7.55
Co-codamol 15mg/500mg tablets
100
£3.11
Co-codamol 30mg/500mg capsules
100
£5.70
Colecalciferol 1,000unit capsules
30
£5.89
Digoxin 125microgram tablets
28
£2.63
Digoxin 250microgram tablets
28
£2.51
Digoxin 62.5microgram tablets
28
£2.78
Docusate 50mg/5ml oral solution sugar free
300
£17.75
Donepezil 5mg tablets
28
£1.60
Dorzolamide 20mg/ml / Timolol 5mg/ml eye drops 0.2ml unit dose preservative free
60
£24.03
Dorzolamide 20mg/ml eye drops
5
£3.55
Duloxetine 20mg gastro-resistant capsules
28
£7.52
Duloxetine 30mg gastro-resistant capsules
28
£1.98
Duloxetine 40mg gastro-resistant capsules
56
£11.25
Duloxetine 60mg gastro-resistant capsules
28
£3.06
Escitalopram 10mg tablets
28
£1.22
Escitalopram 20mg tablets
28
£1.43
Escitalopram 5mg tablets
28
£2.50
Esomeprazole 40mg gastro-resistant capsules
28
£2.81
Estradiol 10microgram pessaries
24
£9.40
Estriol 1mg/g vaginal cream
15
£5.36
Etoricoxib 30mg tablets
28
£4.03
Etoricoxib 60mg tablets
28
£11.89
Etoricoxib 90mg tablets
28
£11.42
Ezetimibe 10mg tablets
28
£10.10
Famotidine 40mg tablets
28
£28.37
Fenofibrate micronised 160mg tablets
28
£3.28
Haloperidol 1.5mg tablets
28
£3.23
Haloperidol 5mg tablets
28
£4.35
Irbesartan 300mg tablets
28
£1.25
Isotretinoin 20mg capsules
30
£9.83
Ketoconazole 2% shampoo
120
£17.51
Lactulose 3.1-3.7g/5ml oral solution
500
£3.38
Latanoprost 50micrograms/ml / Timolol 5mg/ml eye drops
2.5
£6.01
Lofepramine 70mg tablets
56
£18.05
Mebeverine 200mg modified-release capsules
60
£6.42
Memantine 10mg tablets
28
£1.20
Memantine 10mg/ml oral solution sugar free
50
£42.50
Memantine 20mg tablets
28
£1.60
Metformin 1g modified-release tablets
56
£3.19
Metformin 500mg modified-release tablets
56
£1.74
Methylphenidate 10mg tablets
30
£2.98
Metoclopramide 10mg tablets
28
£1.09
Midazolam 10mg/2ml solution for injection ampoules
10
£5.33
Mometasone 0.1% ointment
30
£3.38
Mometasone 50micrograms/dose nasal spray
140
£9.20
Montelukast 10mg tablets
28
£1.81
Morphine sulfate 10mg/1ml solution for injection ampoules
10
£8.03
Mycophenolate mofetil 500mg tablets
50
£6.50
Nebivolol 2.5mg tablets
28
£10.96
Nebivolol 5mg tablets
28
£6.00
Nortriptyline 10mg tablets
100
£2.33
Olanzapine 10mg tablets
28
£1.04
Ondansetron 4mg tablets
10
£6.06
Oxycodone 10mg/1ml solution for injection ampoules
5
£6.05
Paracetamol 500mg effervescent tablets
100
£5.57
Pregabalin 150mg capsules
56
£2.07
Pregabalin 200mg capsules
84
£4.50
Pregabalin 300mg capsules
56
£3.08
Pregabalin 50mg capsules
84
£24.00
Primidone 250mg tablets
100
£88.02
Prochlorperazine 3mg buccal tablets
50
£8.43
Propantheline bromide 15mg tablets
112
£100.61
Pyridostigmine bromide 60mg tablets
200
£17.44
Quetiapine 100mg tablets
60
£2.15
Quetiapine 150mg tablets
60
£2.74
Quetiapine 200mg tablets
60
£3.19
Quinine sulfate 200mg tablets
28
£7.05
Quinine sulfate 300mg tablets
28
£3.10
Rasagiline 1mg tablets
28
£36.00
Risperidone 2mg tablets
60
£1.92
Risperidone 4mg tablets
60
£2.22
Rizatriptan 10mg tablets
3
£6.67
Ropinirole 2mg tablets
28
£26.95
Ropinirole 500microgram tablets
28
£15.00
Simvastatin 20mg tablets
28
£1.04
Sodium chloride 0.9% nebuliser liquid 2.5ml unit dose ampoules
20
£9.50
Sodium valproate 200mg/5ml oral solution sugar free
300
£11.85
Tacrolimus 0.1% ointment
60
£35.28
Tamsulosin 400microgram / Dutasteride 500microgram capsules
30
£11.50
Temazepam 10mg tablets
28
£24.19
Terbinafine 1% cream
30
£6.87
Terbinafine 1% cream
15
£3.44
Trospium chloride 20mg tablets
60
£12.24
Zolmitriptan 2.5mg tablets
6
£16.45
Zolmitriptan 2.5mg tablets
12
£32.90
Zonisamide 100mg capsules
56
£23.31
Zonisamide 50mg capsules
56
£23.57
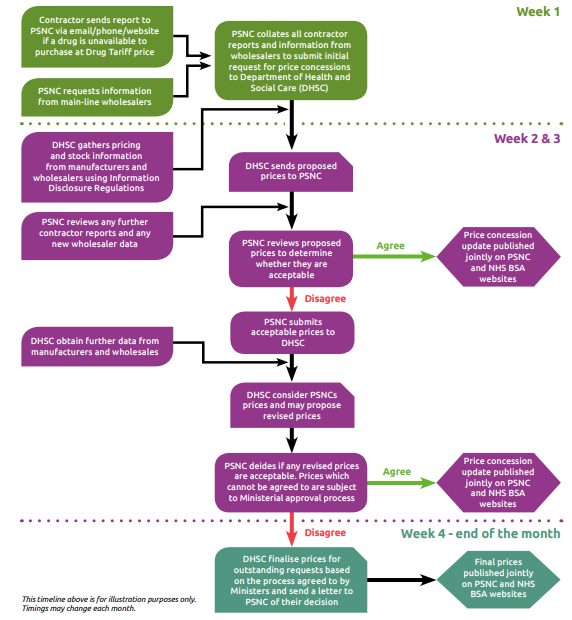
Background
Each month, Community Pharmacy England receives a considerable number of contractor reports (submitted via an online reporting form, by email or telephone) and system-generated reports showing actual purchases of generics made by community pharmacies above the Drug Tariff listed price. We also monitor monthly price lists and price change notifications shared by various suppliers. The reports received help us investigate and determine whether it is appropriate to submit a request for a price concession. At the start of each month, an initial application is submitted to DHSC for any generic drugs reported to us that are unavailable at or below the Drug Tariff price for that month. Further price concession requests for other generic drugs unavailable at Tariff-listed prices are submitted throughout the month. Contractors should continue to use our online reporting form to share details of drugs unavailable at Drug Tariff listed prices. As stock levels and prices can vary across the country, we rely on these contractor reports which help feed into our market surveillance and inform our discussions with DHSC. The reports help us to demonstrate the scale of the problems to DHSC and support escalations on particular lines, as needed. In certain circumstances, DHSC may request wholesaler invoices showing actual purchase prices as evidence to help us make further representations to the Department for an improved price concession. Contractors can email in copies of wholesaler invoices to concessions@cpe.org.uk. Upon receiving Community Pharmacy England’s initial request, DHSC conducts its own research into the market, by gathering volume and price information of drugs from manufacturers, wholesalers and importers, through its data-gathering powers afforded by the Information and Disclosure Regulations 2018 (Information Regulations) Following their initial investigation, DHSC may then decide to grant an initial price concession or grant no price concession at all. DHSC include a margin uplift for drugs granted a price concession. We compare DHSC’s initial proposed price, where offered, against the reported contractor purchase prices, latest wholesaler prices and any further market data gathered after the initial application. The data is used to determine a minimum acceptable price below which a price concession cannot be agreed to. Where we cannot accept a price proposal, we will approach DHSC and seek to negotiate a more acceptable price for contractors. Care is taken to ensure that the prices requested by ourselves are fair and reasonable based on the available purchase data and wholesaler selling-out prices across the country. If we request inflated prices there is a risk of over-delivery of margin which would be subsequently clawed back by DHSC through Category M adjustments. For this reason, we urge contractors to only report actual purchase prices rather than the highest available prices from various wholesalers. DHSC will consider our revised price proposals, and any new requests submitted throughout the month. DHSC will review its initial price offering against our requested prices and the data they have access to. DHSC may then decide to: agree a price concession that matches our initial request, or offer a lower price concession to our initial request; or grant no price concession at all. Any drugs which we are unable to agree prices for are subject to further discussion with DHSC. If we are still unable to come to an agreement on the final price, DHSC, following Ministerial approval, will impose a price they feel is reflective of the market data they have access to. DHSC often cites that the reason behind its decision is due to their research indicating that a considerable amount of stock has been available at or below the proposed price concession at some point during the month in question. We are mindful of the need to finalise price concessions as early as possible in the month so that contractors have certainty over what they will be reimbursed. However, there is a fine balance between agreeing to a price too early in the month versus holding out to secure a better price later in the month particularly in a market where prices are increasing. Price concessions are published on our website as soon as they are finalised with DHSC. Contractors can sign up to receive email alerts as soon as any price concession announcements are made. We understand the difficult challenges faced by contractors when the final prices granted or imposed by DHSC fall below the purchase prices they have paid. This can have a disproportionate effect particularly on those pharmacies dispensing large volumes of any affected lines. As part of the CPCF year 4 (2022/23) and 5 (2023/24) deal for, it was announced that an urgent review of the price concession system would take place. Since September 2022, we have been working with DHSC officials to determine improvements to the price concession system. This led to a six-month review of the concession price system undertaken between August 2022 and January 2023. One of the improvements agreed between Community Pharmacy England and DHSC was that all products granted a concessionary price will be classified as ‘Discount Not Deducted’ for the month(s) in which they are on concession. From 1 April 2023, the following Group Items category was added to Part II of the Drug Tariff for ‘Drugs for which discount is not deducted’: ‘Concessionary Price products (applicable only for the dispensing month(s) for which the product is on concession)‘ As part of a package of measures developed to improve the price concession system, the DHSC implemented a process to roll over certain concessionary prices to the following month. From May 2023, the roll over process applies to any products where Community Pharmacy England has submitted the request later in the month (on or after the 23rd of the month). However, this process would NOT apply to all price concessions granted on or after the 23rd of the month. Any rolled over prices can be adjusted upwards if we receive reports from our pharmacies to indicate that suppliers’ selling prices have increased. The review can be requested at any point during the month. In March 2024, it was announced that from April 2024 a retrospective top-up payment system for price concession lines will be implemented. This is an outcome from the Price Concession Review, which was agreed as part of the 2022/23 and 2023/24 funding settlement for Community Pharmacy. This was a major review of price concession systems, conducted in collaboration between Community Pharmacy England and DHSC. For more information on retrospective top-up payments, click here. As part of the margins survey each year, an exercise is conducted to calculate the financial impact of concession lines on contractors throughout the year. Data is gathered for all items which have been on concession and the financial impact of these lines is calculated and accounted for in the final margins survey result. The calculation also considers any discount deduction (‘clawback’) applied to reimbursement of concession lines and any wholesaler surcharges paid by contractors. If an independent contractor selected in the survey sample purchased a drug at price higher than the final price concession, this will be picked up by the margins survey. This is then factored into the overall retained margin survey result and is off set against any excess margin made on other sampled drugs. On the contrary, if the price concession is higher than the contractor’s actual purchase price, any margin earned will count towards the community pharmacy’s overall retained margin allowance of £800m per year. Q. Why does it take so long for prices to be released? A. The reason for the delays in announcement of concessions is because we see prices for many lines changing throughout the month and the initial prices granted by DHSC are not reflective of the reports we have received from contractors. We understand that contractors want certainty of expected reimbursement prices and that prices granted late in the month are not ideal, especially when there is a wide variation between Tariff and purchase prices. However, we do need to strike a fine balance between agreeing to a price too early in the month versus holding out to secure a better price later in the month particularly in a market where prices are volatile. Q. How long do price concessions last? A. If a price concessions is granted, it is valid until the end of the month in which it was granted*. Community Pharmacy England needs to apply/re-apply for concessions on a monthly basis. If there is an on-going supply problem, it is possible that a new concession will be granted by DHSC the following month; however, this is not guaranteed. *From May 2023, certain prices may roll over where Community Pharmacy England has submitted the request later in the month (on or after the 23rd of the month). However, this process would NOT apply to all price concessions granted on or after the 23rd of the month. Q. Why do some drugs take longer than others to be added to the list of price concessions granted? A. A price concession for some products can be agreed early where prices are acceptable to Community Pharmacy England based on the reports and data received. Other drugs may be requested later in the month or require further negotiation and interrogation based on contractor reports and wholesaler prices before an agreement can be reached. If agreement cannot be reached the decision is made by ministers to either impose a concession price or grant no concession at all Q. Why do pharmacies need to report generic pricing issues each month? If a price concession is granted in one month and is still a problem in the next month why doesn’t the price roll over? A. Typically, price concessions only apply for the month in which they are granted. Because the market fluctuates on a regular basis in terms of stock levels and prices it would not usually be appropriate to roll the price over from one month to the next. However, from May 2023, certain prices may roll over where Community Pharmacy England has submitted the request later in the month (on or after the 23rd of the month). However, this process would NOT apply to all price concessions granted on or after the 23rd of the month. We regularly monitor the market through contractor reports and communications with wholesalers. Where appropriate, we apply to DHSC for price concessions on products which aren’t available at Drug Tariff price. As stock levels and prices can vary across the country, we rely on contractor reports to help feed into our market surveillance and our discussions with DHSC. It is also important to note that the Department may not act on something unless contractors have reported it. Q. Why aren’t price concessions granted on the first day of each month? A. If there is a pricing issue, Community Pharmacy England needs to make a fresh concession application at the start of each month. DHSC then take time to undertake checks and make a decision. In some cases, there is a need for negotiation between Community Pharmacy England and DHSC on an individual product’s circumstances; this can take time. We would like to see changes to the arrangements that would allow contractors to have certainty over what they will be reimbursed, much earlier in the month, a point which we continue to raise with the DHSC. From May 2023, certain prices may roll over where Community Pharmacy England has submitted the request later in the month (on or after the 23rd of the month). Therefore, these prices will be visible from the start of the month they are rolled over into. However, this process would NOT apply to all price concessions granted on or after the 23rd of the month. Q. Are all prices announced on or after the 23rd of the month rolled over to the following month? No. Prices are rolled over to the following month for products where a price concession request is submitted late in the month (on or after the 23rd of the month) and where the final prices granted were agreed to by Community Pharmacy England. Not all price concessions published late in the month roll over to the following month. This may be either because they were requested prior to 23rd of the month or the final prices were imposed by DHSC. Q. Can rolled over prices be adjusted after they are published if purchase prices increase mid-month? A. Yes. Rolled over prices can be adjusted upwards if Community Pharmacy England receives reports from pharmacy owners showing an increase in purchase prices. The review can be requested at any point during the month. Q. When are rolled over prices announced? A. All agreed rolled over prices are published on Community Pharmacy England’s website on the first working day of each month. There may be months’ where no prices roll over to the following month either because all concessions were requested prior to 23rd of the month or the final prices were imposed by DHSC. Q. What happens if a price concession is announced after the date that I have sent my EPS claim message to the NHS Business Services Authority (NHSBSA) for an EPSR2 prescription? A. Price concessions, once granted, apply for the whole ‘dispensing month’. For example a price concession announced on August 30th applies to the entirety of your August ‘prescription bundle’. Your August prescription bundle is made up of all your paper prescriptions which are sent to the NHSBSA by the 5th September and all the EPS prescriptions which fall into the August dispensing month (see below). Prescriptions for any one dispensing month are not priced until the NHSBSA receives both the electronic and paper prescriptions as the MYS submission figures are needed to calculate the advance payment for the contractor. Q. Why can’t Community Pharmacy England tell where they can see stock at the agreed price? A. Community Pharmacy England is unable to advise contractors where to procure products from due to competition law. The French equivalent of Community Pharmacy England, Ordre National des Pharmaciens, was fined €5 million by the European Commission for exactly this behavior. Q. Can price concession drugs be exempted from discount deduction? A. Yes, from 1st April 2023 all concession lines will be considered as Group Items for Discount Not Deducted and a new category was introduced into Part II of the Drug Tariff ‘Drugs for which discount is not deducted’. Q. I have received a prescription for ’28 x 5mg tablets’; however, there is currently a supply issue with that strength and we can only purchase it above Drug Tariff price. The 2.5mg strength is available and works out at the same Drug Tariff price so can I dispense ’56 x 2.5mg tablets’ instead? A. No. Reimbursement will be based on the prescribed strength and quantity (Please note that the ‘PC’ endorsement is not a sufficient endorsement in this situation). If contractors believe it is in the patients best interest to ‘double up’ to support patient care, contractors are advised to return the prescription to the prescriber so they can make a clinical decision and if necessary amend the prescription to ensure correct reimbursement. Q. I have been told by my wholesaler that a Part VIIIA licensed generic product is unavailable. There is not an alternative proprietary product available but a specials manufacturer can prepare this product for me. Can a price concession be requested? A. No. DHSC’s view is that the prescription should be referred back to the prescriber so that they have the opportunity to prescribe an alternative licensed product and/or are aware of the changes in liability caused by an unlicensed product being given to the patient. If the prescriber believes that the product should be specially manufactured, the prescription should be amended to specify “unlicensed special” within the product description. If the prescriber has stated the name of the specials manufacturer, the NHSBSA will pay based on the endorsed invoice price for the specially manufactured product rather than the Drug Tariff Price. Remember that if the prescriber makes a hand written amendment or includes additional product information that does not appear in the product description (i.e. to provide a certain brand), the prescription must be included in the red separator. It is helpful to inform the Community Pharmacy England’s Dispensing and Supply Team about the shortage. If there is a long term supply problem, we can make an application to DHSC to remove the product from the Drug Tariff. More information on the dispensing of unlicensed medicines is available in this section of our site. Q. I have received a prescription for a Part VIIIB unlicensed medicine but cannot obtain it at the Part VIIIB price, what should I do? A. Pharmacy contractors should ensure they have considered a range of suppliers and where they are still having difficulties, pharmacy contractors should contact Community Pharmacy England who will then be able to investigate the situation and apply to the DHSC for a price concession if appropriate. Q. What additional endorsements are needed for products only available above the Drug Tariff price? No additional endorsements are required on prescriptions for medicines that are granted a price concession as the NHSBSA automatically reimburses pharmacies based on the concessions granted by DHSC for a particular month. There is no need for pharmacies to endorse the invoice price paid or the initials ‘NCSO’ – this endorsement has not been in use for reimbursement of price concessions since April 2013 How the price concession system operates Price concession webinar now available on demand Medicines Supply and Price Concessions Resources Medicines Supply Factsheet (July 2022) Contractor Update on Medicines Supply (July 2022) BGMA Best Practice Guidelines on Notification of Medicine Shortages
Additional Information