Repeat Dispensing and eRD
Published on: 10th July 2013 | Updated on: 17th April 2024
At least two thirds of all prescriptions generated in primary care are for patients needing repeat supplies of regular medicines and since 2005, Repeat Dispensing has been an Essential Service within the Community Pharmacy Contractual Framework (CPCF).
Patients using the service obtain repeat supplies of NHS prescriptions without the need for their GP practice to issue a prescription each time a supply is required.
The service was designed to save GP practices and patients time and improve convenience and access to prescriptions, by allowing community pharmacy teams to take a more active role in the process of safe supply of patients’ regular prescriptions.
Under the repeat dispensing service pharmacy teams will:
- Dispense repeat dispensing prescriptions issued by a general practice;
- Ensure that each repeat supply is required; and
- Seek to ascertain that there is no reason why the patient should be referred back to their general practice.
Originally this service was carried out using paper prescriptions, but as the Electronic Prescription Service (EPS) has developed, the majority of repeat dispensing is now carried out via EPS release 2 and is termed electronic Repeat Dispensing (eRD). eRD is much more efficient and convenient for all involved.
Click on a heading below for more information.
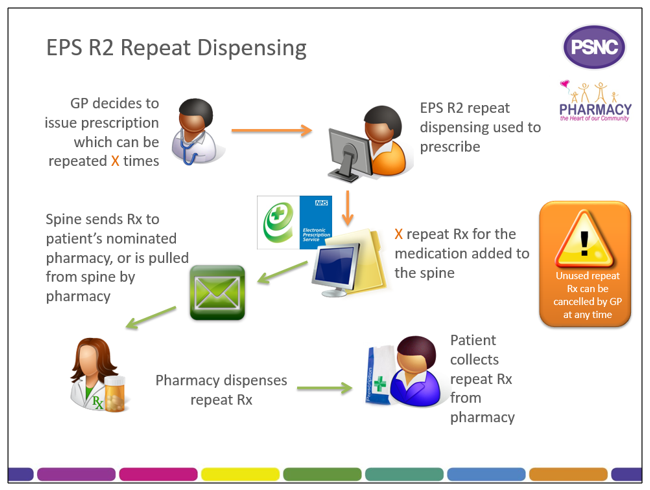
eRD is a process that allows a patient to obtain repeat supplies of their medication or appliances without the need for the prescriber to issue repeat prescriptions each time.
When issuing a repeatable prescription using eRD, the prescriber will authorise a prescription with a specified number of ‘issues’; each issue contains the same prescribed items. eRD allows the prescriber to electronically authorise and issue a batch of repeat prescription issues for use for up to 12 months.
When a prescriber issues an eRD prescription series using their EPS Release 2 prescribing system, in addition to the information found on a standard EPS prescription, the eRD message contains:
- The intended interval between each issue; and
- How many batch issues there are.
The prescription issues are then made available electronically for dispensing at the specified interval by the patient’s nominated pharmacy.
When issuing an eRD repeatable prescription batch, prescribers can issue a Repeatable Prescription Authorising Token to the patient, but the patient does not need one to be able to collect their eRD prescription from their nominated pharmacy.
The contractual requirements for all Essential services are set out in the The National Health Service (Pharmaceutical and Local Pharmaceutical Services) Regulations 2013, but the following service specification was published in 2004 to provide a plain English version of the requirements. It does not mention eRD as its publication was prior to eRD being introduced.
Repeat Dispensing Service Specification
When will the pharmacy receive each issue?
The Spine manages the release of each individual batch issue. The first batch issue is available as soon as it is received by the Spine; subsequent issues will be created on the Spine ready to be pulled down later.
To allow pharmacy teams to prepare items for dispensing in advance of a patient visiting the pharmacy, the Spine will automatically send the nominated pharmacy a repeat dispensing batch issue seven days before the expected end date of the previous batch issue.
It is possible for a pharmacy to pull down issues in advance of them being sent automatically from the Spine, for example, where the instalment dispensing interval is flexible and the pharmacist believes that an instalment should be dispensed at an earlier time because the patient is going on holiday. Pharmacy IT system suppliers also have flexibility to implement more advanced scheduling functionality in their systems to support workflow in pharmacies.
What do pharmacy teams need to do before issuing medicines to the patient?
Before issuing the prescription to the patient, pharmacy teams need to establish that the patient is taking or using their medication appropriately and that there are no reasons why the medication in question should not be supplied.
This can be undertaken by asking the patient or their representative several questions. These questions are detailed in the following Community Pharmacy England Briefing, which also includes points which can be used to promote eRD to patients.
Community Pharmacy England Briefing 004/17: eRepeat Dispensing – A factsheet for pharmacy teams
What happens after the last issue is dispensed?
A new repeatable prescription series can be requested after the last issue is dispensed. Once all authorised issues have been dispensed or if remaining batch issues have ended, the repeatable prescription series is complete and the patient must contact their GP practice to arrange for another repeatable prescription series to be issued.
Pharmacy teams should advise patients of the need to contact their prescriber when dispensing the last issue of a repeatable prescription series.
Patient’s ability to change nomination between issues
A repeatable prescription series can only be issued electronically where it is being sent to a patient’s nominated pharmacy. Patients can choose to change their nominated pharmacy before the end of the repeatable prescription series. In this case, all outstanding issues which have not been downloaded will be transferred to the new nominated pharmacy. This is different from paper prescription-based repeat dispensing system where all issues must be obtained from the same pharmacy.
How do I endorse, submit and reconcile eRD prescriptions?
Visit our endorsing, submission and reconciliation (EPS) hub page for information on those topics.
All eRD prescription issues must be claimed within 365 days of the prescriber’s original signature date because eRD issues cannot be submitted beyond that point.
Training
All pharmacists working in community pharmacies will need to understand how eRD works and the following training programmes can help with developing that knowledge.
CPPE repeat dispensing e-learning and e-assessment
NHS Digital eRD learning modules for pharmacy teams and GP practice teams
Pharmacy staff also need to understand how to operate eRD using their Patient Medication Record system; training and guidance on this will be available from IT system suppliers.
eRD prescriptions are processed in a similar way to other EPS prescriptions; this website includes EPS guidance on the following topics:
Endorsing and submission within EPS
Resources
Community Pharmacy England Briefing 004/17: eRepeat Dispensing – A factsheet for pharmacy teams
One side of this briefing contains key phrases to help advise patients on the benefits of eRD, whilst the other side provides a list of questions to ask patients collecting a repeat dispensing/eRD prescription.
Claiming for EPS prescriptions on time factsheet
In this factsheet we explain how to reduce the risk of delays to payment caused by timing issues with electronic prescriptions.
EPS and eRD statistics (including links to statistics with eRD percentage at GP practice and Integrated Care System level)
Wessex AHSN eRD website section has lots of support materials, including videos, animations and an eRD Handbook.
eRD Factsheets
The benefits of eRD to patients, GP practices and pharmacies
Working with GP practices to roll out eRD and optimise its use
Business change workshop actions list template (Microsoft Word)
Repeat medicines synchronisation form (Microsoft Word)
NHSBSA eRD resources and guidance for GP practices
NHS Digital eRD resources and guidance for GP practices
The Wessex AHSN eRD website section has lots of support materials, including videos, animations and an eRD Handbook.
The regulations state that:
Pharmacy owners must ensure that appropriate advice about the benefits of repeat dispensing is given to any patient who:
(i) has a long term, stable medical condition (that is, a medical condition that is unlikely to change in the short to medium term), and
(ii) requires regular medicine in respect of that medical condition, including, where appropriate, advice that encourages the patient to discuss repeat dispensing of that medicine with a prescriber at the provider of primary medical services whose patient list the patient is on.
This means that pharmacy teams need to identify appropriate patients and provide them with information about eRD, with the aim that there is an increase in the use of the service by patients. Appropriate advice can be given to patients in a number of ways such as:
- Verbally explaining about the service and its benefits to patients; and
- Providing patients with a leaflet describing the service when they are collecting a prescription.
The following materials can be used to promote eRD to patients:
Community Pharmacy England eRD poster (PDF)
Community Pharmacy England eRD poster (Word)
Community Pharmacy England eRD leaflet (PDF)
Community Pharmacy England eRD leaflet (Word)
NHSBSA eRD poster and flyer for use in pharmacies
NHSBSA eRD webpage for patients (including an animation)
Community Pharmacy England Briefing 004/17: eRepeat Dispensing – A factsheet for pharmacy teams
This factsheet contains key phrases to help advise patients on the benefits of eRD.
If pharmacy teams identify patients who are suitable for eRD, with the patient’s consent, they could refer the patient to their GP practice. The following referral form can be used to make such a referral:
Repeat dispensing/eRD referral form (Word eForm for electronic completion)
Repeat dispensing/eRD referral form (for completion by hand) (Word)
Repeat dispensing/eRD referral form (for completion by hand) (PDF)
Social media tweet templates for pharmacy teams
The following template tweets can be adapted (the square bracketed text) to promote eRD to local patients via Twitter:
- Pharmacies in [local area] can offer you a way to collect your repeat prescriptions through electronic repeat dispensing #eRD without you having to order them from the doctor. Ask your local pharmacist / doctor for details #thinkpharmacy;
- Did you know electronic repeat dispensing #eRD to manage your repeat prescriptions is available from [your/our] pharmacy? Pop in and ask [your local pharmacy team/us] about eligibility #thinkpharmacy;
- Patients often don’t have the time to order their prescriptions from the doctor. Electronic repeat dispensing means you don’t have to order from the doctor #eRD #thinkpharmacy; and
- [Percentage number] patients had their repeat prescriptions dispensed through electronic repeat dispensing #eRD within [local area ICS] last year – how do you get your repeat prescriptions? #thinkpharmacy.
Q. Is patient consent required to use eRD for their repeat prescriptions?
No. Prior to the COVID-19 pandemic, explicit patient consent had been required for eRD, however patient consent requirements for eRD were suspended during the pandemic to encourage wider use of eRD. The changes were made permanent on 1st October 2021 following changes to the NHS (General Medical Services Contracts) Regulations 2015.
Q. How long does a repeat dispensing prescription remain legally valid?
A Repeat Dispensing/eRD prescription for a non-Controlled Drug has to be dispensed for the first time within six months of the ‘appropriate date’, with subsequent issues valid for 12 months from the signed date. The ‘appropriate date’ is the later of either the date the prescription was signed or the date indicated as the start date.
For example, consider a prescription for Salbutamol 100micrograms/dose inhaler requesting 12 months supply, split amongst twelve batch issues (RD forms). If the signed date on the prescription (RA form) is 1st January 2024, then if the patient is to obtain all of their medicine, the pharmacy must have dispensed fully within one year, and therefore by 31st December 2024.
Schedule 2 and 3 Controlled Drugs cannot be prescribed on repeat dispensing prescriptions.
Repeat dispensing prescriptions for Schedule 4 Controlled Drugs must be dispensed for the first time within 28 days of the appropriate date with subsequent issues valid for 12 months from the signed date. Repeat prescriptions for Schedule 5 Controlled Drugs are treated the same as non-Controlled Drugs and must therefore be dispensed for the first time within six months of the appropriate date with subsequent issues valid for 12 months from the signed date.
Q. The prescriber has not identified a dispensing interval on the prescription and the patient has told me that they are going on holiday for six weeks. Can I dispense two issues at the same time for the patient?
Yes, where a prescriber has not indicated the interval, the pharmacist should use their professional judgement to dispense instalments at an appropriate interval. From a process perspective, the issues must be pulled down and dispensed in order, so the pharmacy would be required to pull down the first issue, update the Spine to indicate that the issue had been dispensed and then repeat the process with the second issue.
Q. Are Repeat Authorisation (RA) forms required for electronic repeat prescriptions in the same way that they are for paper repeat dispensing prescriptions?
There is not an EPS ‘RA form’, but the patient may present the ‘repeatable prescription authorising token’ (see next question) which is not required to be seen by the pharmacy.
Q. What is the ‘repeatable prescription authorising token’? Are pharmacy teams required to see it for eRD dispensing?
It is a type of prescription token. Prescribers can provide the patient with this so the patient knows how many prescription batch issues have been authorised. Prescription tokens are printed on prescription form stationery by the prescriber to accompany the electronic prescriptions. For standard prescriptions these are occasionally printed at the patient request or to pass on clinical information. With electronic repeat dispensing, prescribers may pass a token to the patient.
Pharmacy teams are not required to see this repeat token to process repeat prescriptions, and where it is presented, there is no need to send it to the Pricing Authority, unless it has been used as an alternative to a dispensing token for one of the dispensing episodes to capture a patient signature.
Q. Why does paper repeat dispensing involve a paper (RA) form, whilst there is no (RA) form for eRD prescriptions?
With paper prescriptions, the legal instruction to a pharmacy to dispense, comes from the hand-written signature. Paper repeat dispensing therefore involves a paper RA form being issued by the prescriber which includes the legal instruction to dispense against the paper RD forms.
Electronic prescriptions use electronic signatures and repeat dispensing is therefore simpler for electronic prescriptions. When issuing a repeatable prescription, the prescriber will electronically authorise a specified number of issues and the patient’s nominated pharmacy can pull these down from the spine, as required.
Q. How is exemption information captured for eRD?
As with standard electronic prescriptions, you must enter the correct payment/exempt category on the electronic prescription using the pharmacy IT system, to ensure reimbursement is correct.
In addition, where a patient signature is required for electronic repeat prescriptions, this must be captured separately for each dispensing issue. If the patient pays for their prescription or is non-age exempt, the pharmacy can print a dispensing token to capture the patient signature. The only tokens required to be sent to the Pricing Authority are those relating to paid prescriptions and those used to collect the patient’s exemption declaration (for a reason other than age) will be required to be sent to the Pricing Authority each month for audit purposes.
Q. How is the dispensing site determined?
A repeatable prescription can only be issued electronically where it is being sent to a patient’s nominated pharmacy. Patients can choose to change their nominated pharmacy before the end of the repeatable prescription. In this case, all outstanding issues which have not been downloaded will be transferred to the new nominated pharmacy. This is different to the procedure for paper-based repeat dispensing, where all issues must be obtained from the same pharmacy.
Q. Can issues be cancelled by the GP and/or pharmacy?
Yes, if the patient’s circumstances do change, the GP can cancel and reissue a repeat prescription for a patient.
An electronic prescription can only be ‘cleanly’ cancelled electronically where it has not been pulled down to a pharmacy IT system. If an electronic prescription has already been pulled down by the pharmacy, prescribers should request that the prescription is not dispensed, for example, telephoning the pharmacy.
Another method of cancellation of a batch issue a pharmacy has not yet processed is where an item or form is marked ‘not dispensed’ and is then submitted to the Pricing Authority. This method of cancellation has the benefit that the prescription will not require to be returned to the Spine, reducing the risk it could inadvertently be dispensed by another pharmacy.
Read more on the Cancelling prescriptions section of the EPS dispensing webpage.
Originally repeat dispensing was mainly carried out using paper prescriptions but as EPS has developed, the majority of repeat dispensing is now carried out via eRD. However, pharmacy owners may still receive paper repeat dispensing prescriptions.
How does repeat dispensing using paper prescriptions work?
Paper repeat dispensing involves the use of a repeatable paper prescription (authorising) form and a number of batch issue paper forms. These forms will be generated by prescribers through their GP practice IT systems.
The repeat authorising form (RA) associated with paper prescriptions should contain prescriber, patient and prescribed medicine details and include the number of issues required and the dispensing interval (e.g. weekly, monthly, quarterly). The form should contain the letters “RA” on the right-hand side of the form. This form must be signed by the prescriber.
Alongside the RA form, a set of batch issue (RD) forms are issued. The maximum number of RD forms that can be issued is equivalent to one year’s duration of prescribing of an individual item.
These forms should contain the same prescriber, patient and prescribed medicine details as the authorising forms and indicate in the prescribing area that the form is being used for repeat dispensing. These forms should include the letters “RD” on the right-hand side.
Batch issue forms should not be signed; however, the prescriber’s signature box should be annotated with text displaying the words Repeat Dispensing: XX of YY, where XX is the number of the batch issue form and YY is the total number of batch issues covered by the repeatable prescription (e.g. 1 of 6, 3 of 12 etc). The date on all of the batch issues and the repeat authorising form will be the same.
Endorsing requirements for paper repeat dispensing prescriptions
Each batch issue form (RD) should be endorsed as required in the Drug Tariff Part II Clause 9. In addition, each RD should be stamped with the pharmacy’s stamp and dated with the date on which the items were dispensed.
There is no requirement to endorse the repeat authorisation form (RA) but community pharmacies are required to maintain records of the dispensing of repeatable prescriptions in order that there is a clear audit trail in place.
Submission process for paper repeat dispensing prescriptions
Batch Issue (RD) forms
Dispensed batch issue forms should be submitted to the Pricing Authority at the end of each month in their correct groups, i.e. exempt or charged groups, within each monthly batch of prescriptions.
The order in which these forms are to be submitted within a batch is detailed in the Pricing Authority’s submission guidance. Initial batch issue forms (i.e. 1 of x) need to be sorted separately from subsequent batch issue forms (ie 2 of x, 3 of x etc), both sorted by doctor surname in alphabetical order.
Batch issue forms which have not been dispensed should not be submitted to the Pricing Authority.
Repeat Authorising (RA) forms
Repeat Authorising forms should be submitted to the Pricing Authority at the end of the month in which all batch issue forms have either been dispensed or the medication is no longer required.
In any month, if any repeat authorisation forms have been submitted, tick the appropriate box on the FP34c Submission Form. There is no need to declare the number of repeat authorisation forms submitted.