Special containers and products requiring reconstitution
Published on: 3rd July 2013 | Updated on: 4th October 2023
There are special requirements and reimbursement arrangements for dispensing those products which are recognised as being packaged in special containers or items requiring reconstitution.
A product is granted special container status in cases where it is not practical to split a pack, for example where the product is sterile or hygroscopic. A product can be classed as special container in its complete original pack size or in its sub-pack size (for example a tablet blister strip).
Where the quantity of a product ordered by the prescriber does not coincide with that of an original pack size and the product is considered a special container (as a complete pack or sub-pack size), contractors are required to supply the special container or combination of containers nearest to the quantity ordered and endorse the prescription form with the number and size of these containers. Where the quantity ordered falls exactly between two containers, contractors should round down, and supply the nearest complete container.
Part II Clause 10 B of the Drug Tariff outlines the following criteria which needs to be met for a product to be granted special container status. Where the quantity ordered by the prescriber does not coincide with the of an original pack and the drug or chemical reagent is:
- Sterile
- Effervescent or hygroscopic, or
- a. Liquid preparations for addition to bath water
- b. Coal Tar preparations
- c. Viscous external preparations
- Packed in a castor, collapsible tube, drop-bottle, pressurised aerosol, puffer pack, roll-on-bottle, sachet, shaker, spray, squeeze pack, container with an integral means of applications or any other container form which it is not practicable to dispense the exact quantity
- Semisodium Valproate, Sodium Valproate, Valproic Acid **:
The contractor shall supply in the special container or containers the quantity nearest to that ordered and endorse the prescription form with the number and size of those containers. Where the quantity ordered falls exactly between two containers, contractors should round down, and supply the nearest complete container.
** The Human Medicines (Amendment Relating to Original Pack Dispensing) (England and Wales and Scotland) Regulations 2023 mandating whole pack dispensing of semisodium valproate, sodium valproate, valproic acid will come into effect on 11 October 2023.
Payment for the nearest complete pack or number of packs also applies to antibacterial, antiviral or antifungal preparations listed in 5.1, 5.2 and 5.3 (except 5.3.1) of the British National Formulary (BNF), which are for oral administration and requires reconstitution from granules or powder. Part II Clause 13 of the Drug Tariff states that:
“when the quantity reconstituted from an original pack or packs is unavoidably greater than the quantity ordered and it has not been possible for the contractor to use the remainder for or towards supplying against another prescription, payment will be calculated from the Basic Price of the preparation and will be based on the nearest pack or number of packs necessary to cover the quantity ordered”.
Please note that the phrase “necessary to cover” is interpreted as the amount dispensed must allow the patient to complete the full course of medication prescribed. Therefore, if a product matching the above description is required to be reconstituted, contractors will be paid based on number of packs required to cover the quantity prescribed.
Following the recommendations made by the Independent Medicines and Medical Devices Safety Review and the outcome of a recent public consultation on Original pack dispensing and supply of medicines containing sodium valproate, changes to Human Medicines Regulations 2023 come into effect on the 11th October that mandate full pack dispensing of all licensed medicines containing valproate (see exemption in note below).
To align with this, from 1 October 2023, all licensed medicines containing sodium valproate, valproic acid and semisodium valproate were reclassified as special containers so where the quantity on a prescription is not for a quantity in an original pack size or multiple of pack sizes, the nearest number of full packs will be reimbursed (either rounded up or down; rounded down when the quantity prescribed is exactly half way between rounding up or down). Contractors do not need to endorse the prescription, they will automatically be paid for the relevant number of complete packs. The special container criteria outlined in Part II Clause 10B of the October 2023 Drug Tariff was updated accordingly.
The manufacturer’s original packs include specific warnings and pictograms on the labelling, including a patient card, along with the statutory patient information leaflet (PIL) and an additional patient booklet, which highlight the risks of taking valproate while pregnant. Supplying complete packs of medicines containing valproate ensures patients, and particularly women and girls of childbearing potential, always receive the PIL with warnings about taking the medicine while pregnant.
Please note: pharmacists will be able to make an exception to supply complete packs of medicines containing valproate, based on an individual risk assessment to assess the need for a patient to be supplied the medicine in different packaging (for example in a monitored dosage system) and provided processes are in place to ensure a PIL is supplied.
For more information on the Original Pack Dispensing (OPD) and Valproate regulation changes, please refer to our briefing: https://cpe.org.uk/briefings/draft-regulations-for-original-pack-dispensing/
List of all UK licensed products containing valproate to be dispensed in complete packs (manufacturer’s original packs)
| Generic drug name (dm+d descriptor) | Brand and/or Manufacturer | Pack size | Drug Tariff listed line | Special container information |
| Sodium valproate 100mg modified-release granules sachets sugar free | All licensed products | All | Yes | Complete pack (no previous special container status) |
| Sodium valproate 100mg tablets | All licensed products | All | Yes | Complete pack (previously special container at sub-pack level) |
| Sodium valproate 150mg modified-release capsules | All licensed products | All | No | Complete pack (unchanged status) |
| Sodium valproate 1g modified-release granules sachets sugar free | All licensed products | All | Yes | Complete pack (no previous special container status) |
| Sodium valproate 200mg gastro-resistant tablets | All licensed products | All | Yes | Complete pack (previously special container at sub-pack level) |
| Sodium valproate 200mg modified-release tablets | All licensed products | All | Yes | Complete pack (previously special container at sub-pack level) |
| Sodium valproate 200mg/5ml oral solution | All licensed products | All | Yes | Complete pack (no previous special container status) |
| Sodium valproate 200mg/5ml oral solution sugar free | All licensed products | All | Yes | Complete pack (no previous special container status) |
| Sodium valproate 250mg modified-release granules sachets sugar free | All licensed products | All | Yes | Complete pack (no previous special container status) |
| Sodium valproate 300mg modified-release capsules | All licensed products | All | No | Complete pack (unchanged status) |
| Sodium valproate 300mg modified-release tablets | All licensed products | All | Yes | Complete pack (previously special container at sub-pack level excluding Epival which had no previous special container status) |
| Sodium valproate 300mg/3ml solution for injection ampoules | All licensed products | All | Yes | Complete pack (no previous special container status) |
| Sodium valproate 400mg/4ml solution for injection ampoules | All licensed products | All | Yes | Complete pack (no previous special container status) |
| Sodium valproate 400mg powder and solvent for solution for injection vials | All licensed products | All | No | Complete pack (no previous special container status) |
| Sodium valproate 500mg gastro-resistant tablets | All licensed products | All | Yes | Complete pack (previously special container at sub-pack level) |
| Sodium valproate 500mg modified-release granules sachets sugar free | All licensed products | All | Yes | Complete pack (no previous special container status) |
| Sodium valproate 500mg modified-release tablets | All licensed products | All | Yes | Complete pack (previously special container at sub-pack level excluding Epival which had no previous special container status) |
| Sodium valproate 50mg modified-release granules sachets sugar free | All licensed products | All | Yes | Complete pack (no previous special container status) |
| Sodium valproate 750mg modified-release granules sachets sugar free | All licensed products | All | Yes | Complete pack (no previous special container status) |
| Valproic acid 150mg gastro-resistant capsules | All licensed products | All | No | Complete pack (no previous special container status) |
| Valproic acid 250mg gastro-resistant tablets | All licensed products | All | Yes | Complete pack (no previous special container status) |
| Valproic acid 300mg gastro-resistant capsules | All licensed products | All | No | Complete pack (no previous special container status) |
| Valproic acid 500mg gastro-resistant capsules | All licensed products | All | No | Complete pack (no previous special container status) |
| Valproic acid 500mg gastro-resistant tablets | All licensed products | All | Yes | Complete pack (no previous special container status) |
Where the quantity ordered falls exactly between two containers, the rounding down rules do not apply to eligible oral anti-infective products which require reconstitution. This is because where the quantity of a liquid antibiotic, for example, reconstituted from granules or powder, is unavoidably greater than the quantity ordered and it is not possible for the contractor to use the remainder of the reconstituted product to fill another prescription, the contractor should supply enough packs necessary to cover the quantity ordered and will be reimbursed based on the nearest pack or number of packs necessary to cover the quantity ordered.
Products classed as special containers in Part VIII of the Drug Tariff are marked with a small black square (◼) next to the drug name, for example:
![]()
Eligible products for oral administration which require reconstitution from granules or powder are marked with a small black circle (•) next to the drug name in the Drug Tariff, for example:
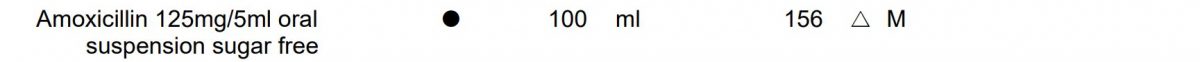
Examples taken from June 2020 Drug Tariff
To confirm the status of a product not listed in the Drug Tariff, you can check the NHS Dictionary of Medicines and Devices browser (dm+d) browser which is updated weekly (every Thursday) by the NHSBSA. Alternatively, dispensing system supplier should automatically prompt users as to which products which are classed as special container and indicate the number of packs (or sub-packs) to dispense. Check with your system supplier on the frequency of dm+d system updates which should ideally be done weekly in line with NHSBSA’s scheduled weekly update. Community Pharmacy England also maintains a special container database which is updated on a monthly basis and can be found at: cpe.org.uk/SCdatabase.
Where the quantity ordered does not coincide with that of the special container pack size you are required to supply the nearest complete pack or sub-pack(s) nearest to the quantity ordered.
In a small number of cases, where there is an over-riding clinical requirement to dispense the exact quantity ordered rather than the nearest complete pack or sub-pack size (for example, if the drug is required to be packaged into a weekly compliance aid of if there is a risk that the patient may misuse the drug), pharmacists would need to assess the clinical appropriateness of splitting the pack to dispense the exact quantity bearing in mind the impact on the stability of the drug once removed from its original packaging. There may be patient safety issues to consider if a pharmacist decides to dispense the exact quantity ordered (if not a multiple of the sub-pack size). For example, if a hygroscopic drug is not stored and used correctly, exposure to moisture could affect the integrity of the drug by potentially decreasing its stability and/or efficacy.
For products classed as special containers, pharmacy teams are required to endorse the prescription form with the number and size of the containers used to dispense from.
With regards to items requiring reconstitution, NHSBSA will automatically flag any eligible items as special containers and therefore reimbursement will be calculated automatically based on the nearest number of packs required to cover the quantity ordered. This will occur even if resulting liquid is not of limited stability as the stability of the liquid does not affect the quantity to be reimbursed.
Pharmacy teams are advised to endorse the amount of packs supplied to enable the patient to complete the full course of medication prescribed.
In cases where there is a clinical requirement to dispense the exact quantity of a product ordered rather than the nearest pack size, to protect against any suggestion of fraud, the contractor should dispense the prescribed quantity and endorse the prescription form clearly with the quantity supplied and the pack size used.
In calculating reimbursement, the special container rules are automatically applied and contractors will be reimbursed for the nearest pack or combination of packs. With regards to items requiring reconstitution, payment will be based on the nearest pack or number of packs necessary to cover the quantity ordered.
There are no specific requirements for sorting these prescriptions separately (unless the prescription meets one of the criteria for inclusion in the red separator).
Q. What quantity would I be reimbursed for supplying against a prescription ordering Prograf 5mg capsules x 40 capsules?
Prograf 5mg capsules are available in a pack size of 50 which is recognised as having special container status. In cases where the prescriber orders a quantity which does not coincide with an original pack and the product has been classed as a special container, the pharmacist should supply a special container or combination of special containers nearest to the quantity ordered. If a prescription calls for 40 Prograf 5mg capsules, you would be reimbursed for dispensing 50 capsules.
Q. I have received a prescription for Ibuprofen 10% gel x 30g but only the 100g pack size is listed in Part VIIIA of the Drug Tariff. What quantity should I dispense and what will I be reimbursed for?
Part VIIIA of the Drug Tariff contains the monthly indicative NHS reimbursement prices for products prescribed generically. Where an item is in Part VIIIA, contractors are reimbursed the price listed against the corresponding pack size (unless a price concession is granted). In this example, only one pack size of Ibuprofen 10% gel is listed in the Tariff (100g pack), even though a variety of other pack sizes exist (30g, 40g and 50g). All available pack sizes are classed as special containers.
For reimbursement purposes, you would be reimbursed for 100g even though a prescription requests 30g. From a legal perspective, you should “supply the special container or containers nearest to that ordered”. Therefore, in this example, even though you will be reimbursed for dispensing the 100g pack size, you should supply the 30g size as ordered. To prevent any allegation of fraud, Community Pharmacy England recommends that the prescription is clearly endorsed with the pack size used.
Q. I have received a prescription for “Amorolfine 5% medicated nail lacquer x 3ml”, what should I dispense and what will I be reimbursed for?
Amorolfine 5% medicated nail lacquer is available in three different pack sizes (2.5ml, 3ml and 5ml) and all pack sizes are classed as special containers. However, Part VIII of the Drug Tariff only lists the 5ml pack size of Amorolfine 5% medicated nail lacquer and therefore for reimbursement purposes, you would be reimbursed the price listed for 5ml pack size even though a prescription requests 3ml. From a legal perspective, you should “supply the special container or containers nearest to that ordered”. Therefore, in this example, even though you are reimbursed for dispensing the 5ml pack size, you should supply 3ml pack size, as ordered. To prevent any allegation of fraud, Community Pharmacy England recommends that the prescription is clearly endorsed with the pack size used.
Q. I have received an FP10 prescription for ‘Erythromycin 40mg/ml / Zinc acetate 12mg/ml lotion x 90ml’. This product is available in packs of 30ml and 90ml; both sizes are listed in the Drug Tariff and are classed as special containers. I have dispensed three packs of 30ml. Is it okay to simply endorse “90ml” on the prescription?
An unqualified number in the endorsement column could be misinterpreted by the NHSBSA, for example if “90ml” is endorsed, the NHSBSA may interpret this as the pack size used and reimbursement could be based on the price of one pack of 90ml rather than 3 packs of 30ml resulting in financial loss to the pharmacy contractor. The recommended endorsement format in this scenario is “amount dispensed/pack size used” (e.g. “90ml/30ml”).
Q. What quantity should I dispense against a prescription ordering Amoxicillin 125mg/5ml oral suspension x 140ml?
The pharmacy would be reimbursed for supplying 2 x 100ml.
As this product falls under Part II Clause 13 of the Drug Tariff – Reconstitution of certain oral liquids, the contractor can round up to dispense the nearest complete container as it is important for patients on antibiotics to cover the complete course of treatment prescribed. Reimbursement for dispensing two packs will be automatic under these rules, but, as is usual with Part VIIIA products, it is good practice to endorse the prescription and declare the total amount dispensed over the pack size used.
Q. What quantity should I dispense against a prescription ordering Heminevrin 250mg/5ml syrup x 100ml?
Clomethiazole 31.5 mg/ml oral solution sugar-free (Heminevrin 250mg/5ml syrup) has been re-classified as a special container as it is not practicable to dispense the exact prescribed quantity where this is different from the original pack size (300ml) or its multiple. The reason for this is that the SPC for Heminevrin 250mg/5ml syrup states the following: ‘Store in the original container. Do not transfer the syrup to another container since it may not be compatible with the container material’. Therefore, for a prescription ordering 100ml of Heminevrin 250mg/5ml syrup, you would dispense and be reimbursed for supplying the complete pack size of 300ml. The patient should be advised to only take the drug for prescribed duration of treatment and return any residual balance to the pharmacy for safe disposal.
Q. I have received a prescription for Lyrica 20mg/ml oral solution x 300ml, what quantity do I dispense?
Lyrica 20mg/ml oral solution comes in a pack size of 473ml which is classed as a special container. As pregabalin is a Schedule 3 Controlled Drug the prescribed quantity of 300ml should be dispensed and the remainder should be safely disposed of. As this product is annotated as a special container, the pharmacy contractor will be reimbursed for the complete pack of 473ml. Due to the nature of the product packaging and the method of its administration, it may be advisable for prescribers to review the prescribing of these products away from split packs, where clinically appropriate.
Q. How do I report a drug which should have special container status but does not have the marker in the Drug Tariff or dm+d?
If you identify any particular product(s) which are not currently classed as special containers but you believe satisfy the criteria (as set out in Part II CLAUSE 10 B of the Drug Tariff), please notify Community Pharmacy England’s Dispensing and Supply Team (0203 1220 810 or info@cpe.org.uk) who will investigate and assess if it meets the relevant criteria. Where appropriate, Community Pharmacy England will make application to the NHSBSA and DHSC seeking for the special container status of the product(s) to be re-determined.
Q. Can products that are unlicensed specials be classed as special containers?
Yes, if the relevant criteria are met, unlicensed specials can be classed as special containers. If a pharmacy receives a prescription for an unlicensed special which has been classed as a special container, the usual special container rules for reimbursement would apply.
Q. Do drugs with a limited stability once opened, automatically qualify as special containers?
Having a limited stability after first opening does not automatically qualify a drug for special container status unless it meets existing special container criteria or is an eligible oral anti-infective drug that requires reconstitution., Due to the nature of products with limited stability, it may be advisable for prescribers to review the prescribing of these products away from split packs, where clinically appropriate.
Q. Do I receive additional fees for dispensing items that have a limited stability following reconstitution?
No additional fees are paid for items that have a limited stability following reconstitution even if multiple containers are supplied to the patient against one prescription in more than one dispensing episode. As only one set of fees can be claimed, it may be appropriate for a contractor to discuss with the prescriber the possibility of providing a separate prescriptions to recognise the requirement to use more than one pack owing to the product’s limited stability.
Q. Can I dispense the exact prescribed quantity if this is different to the special container pack size?
Where the quantity ordered does not coincide with that of the special container pack size you are required to supply the nearest complete pack or sub-pack(s) nearest to the quantity ordered. However, in a small number of cases, where there is an over-riding clinical requirement to dispense the exact quantity ordered rather than the nearest complete pack or sub-pack size (for example, if the drug is required to be packaged into a weekly compliance aid), pharmacists would need to assess the clinical appropriateness of splitting the pack to dispense the
Q. Will my payment be based on endorsement if I dispense the exact quantity ordered of a drug (which is not a multiple of a special container pack size)?
Using their professional discretion, if a pharmacist dispensed the exact prescribed quantity of a product classed as a special container, contractors will be reimbursed according to special container rules, regardless of any endorsement. The payment rules for special containers may mean that in some cases contractors are reimbursed more than the prescribed quantity and, in other cases, it may be less.
Q. I have a prescription for one product out of a combination pack how will I be reimbursed?
Where the prescribed product is only available as part of a combination/titration pack reimbursement will be based on the number of full packs required to fulfil the prescription. Combination / titration packs are considered special containers.
Q. Why have all sodium valproate containing products been reclassified as complete pack special containers?
A. Whole-pack dispensing of medicines containing valproate has been introduced to support the Pregnancy Prevention Programme (PPP) and further decrease the number of babies who are born with serious complications. Sodium valproate a commonly used anti-epileptic (and in some cases the only effective treatment for some patients) is known to be associated with birth defects and neurodevelopmental disorders in children where sodium valproate is taken during pregnancy. To minimise the risk of unborn babies being exposed to the effects of this medication the PPP was introduced in 2018. This further measure of whole pack dispensing has been introduced to ensure that those taking sodium valproate have access to information setting out the risks and need for a woman or girl of child-bearing potential to have a PPP in place before taking sodium valproate. This is because the manufacturer’s original packs include specific warnings and pictograms on the labelling, including a patient card, along with the statutory patient information leaflet (PIL), which highlight the risks of taking the medicine while pregnant.
Q. Can I dispense the exact prescribed quantity if this is different to the special container pack size?
A. Where the quantity ordered does not coincide with that of the special container pack size you are required to supply the nearest complete pack size. For example, where a prescription requests Sodium valproate 300mg modified-release tablets x 28 tablets, you should dispense the nearest complete pack size of 30 tablets. The pharmacy would be reimbursed accordingly as the complete pack of 30 will be treated as a special container by the NHSBSA.
Q. I have received a prescription for Sodium valproate 300mg modified release capsules tablets x 45 tablets. How many packs should I supply?
A. Only one pack of 30 capsules should be supplied. This is in-line with the special container ‘half-way’ rule for special containers which states that, ‘where the quantity ordered falls exactly between two containers, pharmacy teams should round down, and supply the nearest complete container’.
Q.I have received a prescription for Sodium valproate 500mg gastro-resistant tablets x 50 tablets, there are two pack sizes in the Drug Tariff 30 and 100, can I round up to supply 100 tablets?
A. No. For a prescription ordering Sodium valproate 500mg gastro-resistant tablets x 50 tablets pharmacy teams would supply and be reimbursed for the nearest number of complete packs which is 2 x 30 tablets.
Q. Can I supply sodium valproate in a Monitored Dosage System (MDS)?
A. Medicines containing sodium valproate, valproic acid or valproate semisodium must be sold or
supplied in their original outer packaging. However, pharmacists will be able to make an exception to complete-pack dispensing of medicines containing valproate, provided an individual risk assessment is in place that refers to the need for a patient to be supplied the medicine in different packaging (for example in a monitored dosage system) and where processes are in place to ensure the supply of a patient information leaflet (PIL).
Pharmacists will also need to consider the impact on the stability of certain valproate medicines once moved outside their original packaging. For example, Epilim tablets are hygroscopic in nature and therefore sensitive to the effects of moisture. The ‘Special precautions for storage section’ in the SPC states that ‘the tablets should not be removed from their foil until immediately before they are taken’. Any water ingress may result in the chemical and/or physical stability of the drug being compromised.
For further guidance on stability of drugs in Monitored Dosage Systems (MDS) please refer to the Specialist Pharmacy Service guidance on Usage of Medicines in Compliance Aids.
Q. Do the changes apply to all formulations including injectable preparations?
A. Yes. The changes to mandate complete pack dispensing of products containing valproate extend to all licensed formulations of sodium valproate, valproic acid or valproate semisodium. For a prescription ordering Episenta 300mg/3ml solution for injection ampoules x 3 ampoules, pharmacy teams should supply the complete pack of 5 ampoules. The pharmacy would be reimbursed accordingly as the complete pack will be treated as a special container by the NHSBSA.















