Dispensing (EPS)
Published on: 16th July 2013 | Updated on: 7th April 2022
 EPS dispensingy information is set out on this webpage.
EPS dispensingy information is set out on this webpage.
Click on a heading or link to ‘show’ or read more or return to EPS home or EPS submission for more on EPS.
| Top tip | Summary | Advice |
| Split prescriptions | Pharmacists need to work with GPs to have a process in place to be able to identify patients who may have split prescriptions because of the clinical risks. |
Non-EPS item: There are some prescription items that cannot be transmitted electronically, for example it is currently not permitted to issue an electronic prescription for medicine outside the NHS Dictionary of medicines and devices. Patients not being able to obtain all medicines electronically has created problems. Working with GPs: The key lesson is that pharmacists need to work with GPs to have a process in place to be able to identify patients who may have split prescriptions and ensure that affected patients are aware of the risks that nomination could create. Should EPS be used for patient with a non-EPS item? Nomination may not be the best option for these patients. However if you and your local GPs wish to use EPS another option is for prescribers to include a note in the electronic message to indicate that there is also an FP10 for the patient. EPS and controlled drugs: Community Pharmacy England is continuing to call for Schedule 2 and 3 Controlled Drugs to be sent via the service. |
Related resources
Split prescription factsheet (NHS Digital / HSCIC archive pdf):
Issuing an EPS prescription
Prescribing staff can prepare electronic prescriptions, and assign these to a prescriber.
Prescribers can:
- electronically sign prescriptions individually or select multiples to sign in bulk;
- consider appropriate times for signing prescriptions (and may consider adding a recurring diary entry or calendar note to act as reminders at certain times throughout the day).
If a certain prescriber is busy, a prescription can be reassigned to another prescriber by practice staff.
Prescribers electronically sign prescription, causing these to be sent to the central NHS Spine, so that a pharmacy can download (‘pull down’) them.
Prescribers need appropriate Smartcards for their position and roles.
Prescriber type EPS codes
These are explained within the Prescriber type EPS codes factsheet.
Prescriptions have indicators about the type of prescriber that issued the prescription e.g. a General Practitioner (GP) or a Community practitioner nurse. Different prescriber types have different prescribing rights.
With paper prescriptions – the type of paper prescription form stationery, the colour of the form and the description on the form indicate the prescriber type.
EPS electronic prescription messages do not need to make use of the stationery form reference (e.g. ‘FP10SS’) because prescriber type EPS codes are used instead within EPS messages.
Paper prescription form stationery include stationery reference codes e.g. ‘FP10SS’ or ‘FP10HNC’ printed onto the prescription form for use by a prescriber. Paper prescription forms such as ‘FP10SS’ are used to enable printing of paper prescriptions or EPS tokens. These paper forms are explained at: Paper prescription forms.
EPS tokens can still be printed onto relevant paper forms e.g. ‘FP10SS’. NHS Business Services Authority (NHSBSA) scan those EPS tokens submitted during the pricing process, but the standard pricing by NHSBSA makes use of the electronic message not the scanned token.
The EPS prescribing specification explains: “The EPS supports three prescribing models; acute; repeat; and eRD.”
NHSBSA maintains the list of prescriber type EPS codes within the EPS endorsing guide specification: e.g. ‘EPS Prescription Type Code 0001’ indicates “General Practitioner (GP) Prescribing”
EPS prescriptions include prescriber type EPS codes within their electronic message. These prescriber type EPS codes are listed within the EPS endorsing guide. Paper prescription forms such as ‘FP10SS’ used to enable printing of paper prescriptions or EPS tokens are explained at: Paper prescription forms.
Prescribing system suppliers may add logic to their prescribing systems to ensure that prescribers do not inadvertantly try to request an item that they should not e.g. prescribing system logic to prevent a Community practitioner nurse prescriber being able to dispense a controlled drug.
Pharmacy system suppliers may also add logic to their pharmacy systems to prevent pharmacy teams inadvertantly trying to process a prescription which is not allowed to be reimbursed by the NHS. E.g. a pharmacy system could prevent a prescription being processed for a Controlled Drug if the prescriber type EPS code was for a community practitioner nurse prescriber.
Some pharmacy systems suppliers can opt to adjust the colour on-screen when a different prescriber type is indicated, but this is not a pharmacy system requirement.
The EPS spec explains: “The EPS supports three prescribing models; acute; repeat; and eRD.”
EPS was originally rolled out to GP practices. However, EPS continues to be expanded to urgent and other care settings and prescribers.
Read more within Community Pharmacy England’s factsheet:
Medicines Assembly
While the prescription is being prepared and is awaiting collection, there is no requirement to update the prescription status on the Spine.
Accuracy Check
It is for a pharmacy contractor to determine how the accuracy check in the dispensing process is undertaken including when dispensing electronic prescriptions received via EPS Release 2. Options include:
Checking against the prescribed information on screen: Different pharmacy systems will display the prescribed information in different ways on screen. Systems will record both the prescribed information (from the electronic prescription) and the dispensed information (as entered by the user where different from the prescribed item). It will be important for system suppliers to make it very clear to the user when they are viewing prescribed information and when they are viewing dispensed information.
Checking against the prescription or dispensing token: System suppliers have been mandated to print specific information from the electronic prescription message on to the dispensing token without any of this information being edited. This will be assessed by the NHS Digital as part of the clinical safety checks in the Common Assurance process.
Checking against dispensing labels: As dispensing labels may be edited, for example, the dosage instructions changed, there may be a risk to patient safety if an accuracy check is undertaken against dispensing labels. System suppliers may develop other solutions to support accuracy checking for example, printing the electronic prescription information but not on a dispensing token, for example creating a pick list on a smaller slip of paper such as a till receipt to minimise waste.
Prescribers may sometimes erroneously add product information into the prescription dose area.
Prescribers shouldn’t include supplementary product information within the prescription dosage instructions area. A new or paper prescription might be required on occasion.
See also: Avoiding EPS product info in dose area factsheet (Community Pharmacy England pdf one-pager).
Examples: NHSBSA pricing systems cannot see the free-typed information that appear after the dose area. Product info such as ‘Unlicensed‘, ‘brand name‘ or ‘FS‘ (Free supply) should not be free-typed into this EPS space. It does not inform the pricing of the EPS prescriptions.
Why product info shouldn’t be included in the dose area?
If prescriptions are incorrectly prepared with product info within the dose area then: patients, pharmacy teams and NHSBSA may miss the information and not take it into account. A prescriber that is not aware about these issues might try to free-type a note so that it appears after the dosage instructions, e.g. in relation to list/image below.
- Unlicensed items;
- Assorted Flavours – but the ‘Assorted Flavour’ flag should have been used instead.
- ‘FS‘ for Free supply of an item (see the FS and Free of charge sections of EPS Submission webpage)
- A brand/manufacturer or sugar/preservative-free prescription within patient dosage instructions rather than as part of the main name entry of the prescribed product .

Additionally, the pricing implications for erroneously-prepared prescriptions are set out below:
- Standard EPS pricing does not take into account the dose area information. Standard EPS pricing is based on the prescribed product code. NHSBSA can’t use the dose area for initial pricing. Dose area info doesn’t impact the initial payment calculation.
- Paper prescriptions: There is some risk that product info within the dose area might not always be used during the pricing process, even if the paper prescription is submitted within the red separator.
How to request for the prescription to be re-issued?
If pharmacy teams receive a prescription with product info within the dose area, the prescriber should be contacted so that the item can be cancelled and a new prescription generated. The newly-issued prescription should include product info such as within the main-name entry (see image further below).
There will be occasions where a prescriber cannot issue the prescription they wish via EPS for technical reasons. EPS prescribing may not be possible if the item is not listed within either the:
- NHS medicinal item database (dm+d); or
- the GP system*.
*GP suppliers may not always synchronize to every dm+d listing therefore some listings are not ‘selectable’ via EPS. Less common items (e.g. unlicensed ones) are particularly impacted.)
If prescribing systems allow dm+d specified products to be prescribed (as per image below), i.e. ‘Special Order’, ‘Drug Tariff Special Order’, ‘Imported (Country))’ or a manufacturer name appearing as part the main-name entry.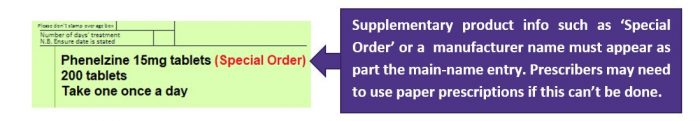
Note: Community pharmacy teams can see what items are listed within dm+d: cpe.org.uk/dmd includes links to dm+d viewers such as the NHSBSA dm+d viewer and OpenPrescribing dm+d viewer.
Having NHSBSA reprice a previously priced prescription (specials only)
Contractors may request NHSBSA recheck specific prescription(s) if ‘specials’ prescriber instructions were followed and the prescription was then priced. Contractors may attach to their recheck application visual evidence of prescriber’s instruction e.g. a screenshot or token copy showing ‘Unlicensed’ was written by the prescriber within the dose area. NHSBSA won’t have been able to initially use that prescriber instruction for pricing, but will be able to adjust the reimbursement after a recheck and repricing of the specials item. Read more at: cpe.org.uk/recheck.
See also
See also: Avoiding EPS product info in dose area factsheet (Community Pharmacy England pdf one-pager) and cpe.org.uk/dosearea.
See the ‘Specials‘ section of this webpage immediately below for a further explanation of scenarios where EPS may be used for special or unlicensed items.
If GP systems allow for some unlicensed items to be prescribed correctly by EPS then these may be processed by the pharmacy. The wording ‘Special Order’, ‘Drug Tariff Special Order’ or ‘Imported (Country))’ needs to be included immediately after the drug name as per the image above.
Prescribers may sometimes erroneously add ‘specials’ information into the prescription dose area e.g. wording such as ‘unlicensed’, ‘special’ or a specials manufacturer appearing after the EPS dose area. Such wording will have been free-typed. If the pharmacy teams receives such an EPS prescription, the team will have to contact the prescriber so that the item can be cancelled and a new prescription generated. NHSBSA cannot use info within the EPS dose area to inform pricing. The newly-issued replacement prescription needs to be on paper or have the product info displayed within the main-name entry.
Note that prescribers can’t always use EPS for unlicensed items for technical reasons such as if the item is not listed within either:
- dm+d; or
- the GP system medicine options (note: prescribing systems reportedly do not always comprehensively incorporate every dm+d listing for the prescriber to be able to select).
As explained, if a prescriber is unable to issue an unlicensed EPS prescription in the correct way, then pharmacy teams should contact the prescriber for a paper FP10 prescription.
Prescribers should not free type ‘unlicensed special’ so that it appears after the dose area (see the ‘Dose area‘ section of this webpage above).
Passing Clinical Messages onto Patients
Release 2 of the Electronic Prescription Service has considerably reduced the flow of paper between the prescriber and the patient so supplementary information must be passed on to patients via alternative routes. Pharmacy teams and local GPs should work together so that all parties agree how non-routine clinical (patient specific regarding medication) information will be communicated from the GP to the patient via the pharmacy team.
Historically, the right hand side of a paper prescription form was used by prescribers to communicate information such as; non-routine clinical information, review dates for patients, an order form for repeat medication and to promote the prescriber’s practice, for example, clinic opening and closing times as well as advertising particular services such as ‘flu clinics’.
However, there is growing concern that the right hand side of a prescription is not always the best option for prescribers to pass on non-routine clinical information to patients. This is because some pharmacists do not automatically see the right hand side of a prescription on their computer screens and can only do so by printing out a dispensing token, which is a practice that is currently being reduced in pharmacy as we move towards a paperless NHS. In addition, the dispensing systems do not always print out the information on the right hand sides of a prescription in an easy to read format, so key information can get lost or hidden.
The long term solution to this issue could be for prescribers to communicate directly with patients using systems such as Patient Facing Services. However, it is recognised that these changes will take time to implement and therefore a temporary solution is required.
Non-routine clinical information (which is specific to a patient and medication item) could be passed from prescribers to dispensers using the ‘message to dispenser’ field on the left hand side. The message content should be concise and appropriate. This should not be used for routine information, but must be specific to the patient.

This method would ensure that pharmacists have visibility of the message when reviewing the left hand side on their computer systems. This information could then be passed on verbally to the patient.
In addition to the above solution, NHS Digital will continue to highlight current inconsistencies with the right hand side of prescriptions within dispensing systems and try to get these issues fixed as soon as possible.
Non-routine clinical information includes:
• Last repeat dispensing batch issue
• Change in dosage of medicine
• End of repeats process – for example, ‘when repeats have run out please contact surgery for a review’
• Details of review appointments, for example, instructions for the patient to arrange an appointment with the prescriber for a blood test where this is non-routine for that patient.
Pharmacy staff will not be required to pass on routine information linked to the services offered by another provider.
Routine information for which pharmacy is not required to pass on includes:
• Patient information: Name, Address, DOB and NHS number
• Doctor’s details
• Repeats: Drug quantity, dose; Last issued date; Next issue date; Review due date (when not within 4 weeks)
• Flu jab
• Check-up: well woman; well man; over 41s; over 75s; obesity; dementia; cancer screening
• Advertisement for patient to take-up a non-essential service at the GP surgery: travel clinic,; physiotherapy; chiropractor; Chiropodist.
• GP opening times
Although GP and pharmacy systems technically can allow this information to be communicated via the right hand side where this is locally agreed by the pharmacy team and the prescriber , there may be more appropriate alternatives. It is recommended that pharmacy teams co-work with their local prescribing organisations to discuss the methods which will be used so that non-routine clinical information is to be passed to patients.
Non-routine information to pass on if the patient requests
Pharmacy staff will also be required to provide the following information to the patient, at the patient’s request:
-
A full list of the patient’s ‘repeat’ medication (as received in the electronic prescription message);
-
The patient’s review date (where greater than 4 weeks); and
-
Information on the number of times the medication can be reordered from the prescriber without a review.
Once the medication has been or is being dispensed the ‘dispense notification’ message can be sent to the central NHS Spine. This does not need to be done immediately after issue; but may be done in accordance with contractor’s chosen processes and system settings; staff might choose a convenient time that fits in with the pharmacy’s business processes and systems. Also, there is some flexibility in how local systems support pharmacists with this process, for example current options within pharmacy systems might send the message at time of labelling, or systems could generate a bar-code to include on the bag label which can be scanned at the point of dispensing to automatically update the system. Some PMR systems may give you the ability to describe a prescription or item as ‘collected’ (which may or may not coincide with sending of an EPS message). At present, different PMR systems are use different terminology about prescription status.
To complete the EPS-related part of the dispensing process, each prescription item must be marked so that the system describes the item as ‘dispensed’ or ‘not dispensed’ (if all prescription items on a prescription have the same status it is possible to mark the entire prescription as opposed to marking individual items). There are also two intermediate statuses that can be used, ‘with dispenser – partial’ and ‘with dispenser – owing’, where part of the prescribed quantity has been dispensed and where none of the prescribed quantity has been dispensed but is likely to be dispensed at a later date. The Spine should be updated after each dispensing event.
We recommend sending EPS messages frequently. In the event of PMR system technical issue (such as a crash causing locally held data to be lost) the impact will be less if relevant EPS messages have already been sent to the NHS Spine.
The EPS central infrastructure supports the recall of dispense notification messages as long as a reimbursement claim message. However, not all pharmacy system suppliers may have built this functionality into local systems. If your system does not support recalling dispense notification messages, we would recommend making a development request to your supplier for this functionality to be added.
Recalling a dispense message is separate from EPS prescription claim amending.
A post-dated EPS prescription will be held locally within the clinical system and will automatically be sent to the pharmacy system on the specified date.
For such prescriptions:
- the content of the prescription cannot be viewed by the pharmacy team meaning prior to the specified date, where the GP surgery is closed, and the patient asks the pharmacy team to check information relating to the prescription, this will not be possible; and
- the medicine cannot be prepared in advance for patients.
Pharmacy teams may wish to work with their GPs to prevent issues. An alternative to use of post-dated EPS prescriptions may be use of eRD.
Dosage area product information
Q. I have received a prescription and see that supplementary product information (e.g. ‘unlicensed’ or brand name) is included within the dose instructions area. Will the prescription be priced correctly and is this prescription valid?
There is the risk that the product information within the dose area will be missed by the patient/pharmacy and NHSBSA: With paper prescriptions it can easily be missed. With electronic prescriptions, pricing will be based on the product code of the prescribed product, therefore, supplementary product information included in the prescriber’s dosage instructions will not be considered when calculating payment. Some prescription should be re-issued by the prescriber onto paper and correctly. See further information in the section ‘Dosage area (avoiding wrong info within)’ on this webpage and also at Avoiding EPS product info in dose area factsheet (Community Pharmacy England pdf one-pager) and cpe.org.uk/dosearea.
Sending Dispense and Claim messages
Q. I have received an electronic prescription but only two of the four items requested are in stock. Can I dispense the items in stock and push the electronic prescription back to the Spine for the patient to get the other two items dispensed at another EPS R2 enabled pharmacy?
No, it is not possible to split the dispensing of items on an electronic prescription form between different pharmacies. This is the same as the arrangements for paper prescriptions. If the patient requires all medicines urgently and is not willing to accept an owing note, an option is to return the electronic prescription to the Spine and provide the patient with a dispensing token so that their electronic prescription can be accessed at another EPS R2 enabled pharmacy.
Q. I have received an electronic prescription from the prescriber and the dosage instructions are not clear, can I manually amend this so that the dispensing label carries the correct instructions?
Yes. Pharmacy system suppliers may design their systems differently but will have the flexibility to allow the dosage instructions to be amended and corrected as necessary by pharmacy staff.
Q. In EPS Release 2, will a prescriber be able to cancel a prescription that has already been dispensed by a pharmacist?
Release 2 of the Electronic Prescription Service will support the cancellation of electronic prescriptions. It will not be possible for a prescription to be electronically cancelled by a prescriber, where it has the following status: ‘With Dispenser’, ‘With Dispenser – Active’, ‘Dispensed’ or ‘Claimed’. Where a prescription cannot be electronically cancelled, a prescriber can follow manual processes, as now, and call the pharmacist to explain that there is a problem with the prescription. To facilitate this, the prescribing system will provide information to the prescriber on which dispensing site has pulled down the prescription.
Q. In my EPS Release 1 enabled system, the system presents me with the quantity and unit of measure prescribed and asks me to confirm, amend or re-key this information. This is laborious. Will I continue to have to do this in Release 2?
No, pharmacists shouldn’t be required to continue to do this in Release 2. To resolve this problem, systems suppliers have been asked to comply with certain standards in the way the quantity of medication and units of measure are expressed in systems. The way systems express this information will be assessed as part of the common assurance process.
Q. I have pulled-down a nominated prescription but do not have the item in stock. The patient wishes to change their nomination settings anyway to a neighbouring pharmacy. If I push the prescription back to the spine and change the nomination settings – will my neighbouring pharmacy be able to access the electronic NHS prescription?
No, when the prescription is returned to the spine it will no longer be flagged as a nominated prescription therefore the prescription ID is required by the other pharmacy to pull the prescription down. A dispensing token should be printed for the patient to take to the other pharmacy.
Factsheets
- EPS CDs FAQs one-page factsheet
- EPS dose area: and exlcuding product info from within it
- Summary of EPS-related forms and tokens
Related webpages
If you have queries on this webpage or you require more information please contact it@cpe.org.uk. To share and hear views about digital developments with like-minded pharmacy team members, join the CP Digital email group today.
Return to the Pharmacy IT hub; EPS home or IT a-z index