Dealing with product information within the dose area
Published on: 12th August 2014 | Updated on: 15th March 2022
Prescribers may sometimes erroneously add product information into the prescription dose area. This webpage for pharmacy teams explains the impact of this, and how to address the problem by requesting a replacement prescription or making use of the NHSBSA recheck process, if needed.
Prescribers shouldn’t include supplementary product information within the prescription dosage instructions area. A new or paper prescription might be needed on occasion.
See also: Dealing with EPS product info in dose area factsheet (pdf one-pager Community Pharmacy England factsheet).
Examples: NHSBSA pricing systems cannot see the free-typed information that appear after the dose area during their standard pricing. Product info such as ‘Unlicensed‘, ‘brand name‘ or ‘FS‘ (Free supply) should not be free-typed into this EPS space. It does not inform the initial pricing of the EPS prescriptions.
If prescriptions are incorrectly prepared with product info within the dose area then: patients, pharmacy teams and NHSBSA may miss the information and not take it into account. A prescriber that is not aware about these issues might try to free-type a note so that it appears after the dosage instructions, e.g. in relation to (see list and image below).
- Unlicensed;
- Assorted Flavours [note: but the ‘Assorted Flavour’ flag should have been used instead].
- ‘FS‘ for Free supply of an item (see the FS and Free of charge sections of EPS Submission webpage)
- A brand/manufacturer or sugar/preservative-free prescription within patient dosage instructions rather than as part of the main name entry of the prescribed product .

Additionally, the pricing implications for erroneously-prepared prescriptions are set out below:
- Standard EPS pricing does not take into account the dose area information. Standard EPS pricing is based on the prescribed product code. NHSBSA can’t use the dose area for standard pricing. Dose area info doesn’t impact the initial payment calculation.
- Paper prescriptions: There is some risk that product info within the dose area might not always be used during the pricing process, even if the paper prescription is submitted within the red separator.
If pharmacy teams receive a prescription with product info within the dose area, the prescriber should be contacted so that the item can be cancelled and a new prescription generated. The newly-issued prescription should include product info such as within the main-name entry (see image further below).
There will be occasions where a prescriber cannot issue the prescription they wish via EPS for technical reasons. EPS prescribing may not be possible if the item is not listed within either the:
- NHS medicinal item database (dm+d); or
- the GP system*.
*GP suppliers may not always synchronize to every dm+d listing therefore some listings are not ‘selectable’ via EPS. Less common items (e.g. unlicensed ones) are particularly impacted.)
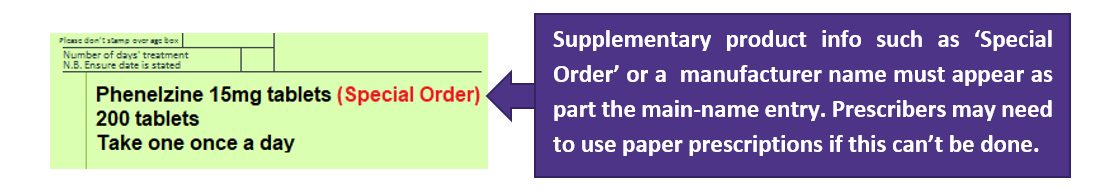
If prescribing systems allow dm+d specified products to be prescribed (as per image below), i.e. ‘Special Order’, ‘Drug Tariff Special Order’, ‘Imported (Country))’ or a manufacturer name appearing as part the main-name entry.
Contractors may request NHSBSA recheck specific prescription(s) if ‘specials’ prescriber instructions were followed and the prescription was then priced. Contractors may attach to their recheck application visual evidence of prescriber’s instruction e.g. a screenshot or token copy showing ‘Unlicensed’ was written by the prescriber within the dose area. NHSBSA won’t have been able to initially use that prescriber instruction for pricing, but will be able to adjust the reimbursement (for ‘specials’) after a recheck and repricing of the item.
Read more at: cpe.org.uk/recheck.
Q. I have received a prescription and see that supplementary product information (e.g. ‘unlicensed’ or a brand name) is within the dose instructions area. Will the prescription be priced correctly (during initial pricing) and is this prescription valid?
There is the risk that the product information within the dosage area will be missed by the patient/pharmacy and NHSBSA: With paper prescriptions it can easily be missed. With electronic prescriptions, pricing will be based on the product code of the prescribed product, therefore, supplementary product information included in the prescriber’s dosage instructions will not be considered when initial pricing is performed. Some prescriptions will need to be re-issued as paper prescriptions. See further information on this webpage.
Q. I am not clear as to whether a prescriber instruction is a proper endorsement (e.g. ‘FS’ or ‘AF’) or whether it has been free-typed?
If the letters appear immediately after the dose area, these have been free-typed. On occasion the prescriber may not have included dose instructions, and the letters appearing appearing after the quantity prescribed could have been free-typed. It is recommended you check the PMR terminal instead of the EPS token. It may be less clear ito check whether the FS/AF flag had been added properly if you only consult the EPS token.
If you are unsure then check with your system supplier or their help materials about the topic.
Further info
Read more at:
If you have queries on this webpage or you require more information please contact it@cpe.org.uk. Note: Community pharmacy teams can see what items are listed within dm+d: cpe.org.uk/dmd includes links to free dm+d viewers such as the NHSBSA dm+d viewer and OpenPrescribing dm+d viewer.
Return to the IT section: EPS home / eRD / Smartcards / System suppliers
Return to the EPS sections relating to: Overview / Preparing&enhancing EPS / Nomination / Dispensing&Supply EPS / CDs / Tokens / EPS/IT contingency planning / Submission / Cancelling/changing EPS / RTEC / Future
EPS resources/factsheets also include: EPS essential checklists, EPS CDs FAQs, checking EPS totals, Nomination Principles, EPS/IT/ODS change, Phase 4, Reporting EPS issues, EPS studies including tips and lessons, Submitting EPS in time, Tokens, Tracker, Upgrading EPS from Release 1 to 2
Ask a question about pharmacy IT: it@cpe.org.uk