Serious Shortage Protocols (SSPs)
Published on: 3rd October 2019 | Updated on: 2nd April 2024
SSPs are a potential way to help pharmacies to manage any serious shortages of medicines that may occur, without needing to refer patients back to prescribers. It is important to note that although legislation permits the issuing of SSPs from 1st July 2019, an SSP will only be considered and issued if there is a serious shortage of a specific medicine.
If, in the Secretary of State for Health and Social Care’s opinion, there is, or may be, a serious shortage of a medicine or appliance then he or she may consult, for instance with medical experts, and decide to issue an SSP. The SSP will specify an alternative product or quantity that may be supplied (an alternative strength or formulation, or generic or therapeutic alternative or less of the product) by community pharmacies. Community pharmacy contractors must consider the SSP and, if, in the supervising pharmacist’s opinion – exercising his or her professional skill and judgment – the alternative product or quantity is reasonable and appropriate for the patient, they may supply the alternative product or quantity (only as specified in the SSP and subject to any conditions in the SSP), provided that the patient consents/agrees to the alternative SSP supply.
Top tips to claim and reconcile payments for SSPs
Active SSPs
| SSP | Expiry Date | Supporting Information |
| SSP059: Monomil® XL 60mg tablets | 3 May 2024 | SSP059 guide |
Details will be updated above as and when changes occur, but the most up-to-date information is available from the NHS Business Services Authority (NHSBSA) website.
Pharmacy teams will be notified of any new SSPs or changes to an existing SSP via NHSmail, the NHS Business Services Authority (NHSBSA) website, via the Community Pharmacy England newsletters, the latest news section of our website and you can also check the Community Pharmacy England Live SSPs page.
To ensure correct payments (fees and reimbursement) are made for any supplies made against an SSP, community pharmacy contractors are required to endorse the paper prescription, or the electronic prescription (where the prescription is issued using the Electronic Prescription Service (EPS)) with the following information:
- SSP [followed by the three-digit reference number applicable to the SSP] – to indicate that the supply was made in accordance with an SSP;
- Details of product supplied in accordance with the SSP (drug name, quantity, strength, formulation, supplier name or brand)*;
- Quantity supplied*;
- Pack size – best practice where multiple pack sizes are available; and
- Invoice price – only if NHSBSA has no list-price available on dm+d.
*The drug details and quantity supplied and endorsed will depend on the relevant SSP.
In most cases, SSP endorsements for specific SSPs are shorter.
In addition, to support pharmacy teams, Community Pharmacy England has also put together some helpful reminders and top tips on the correct SSP endorsement and submission requirements.
General Top tips:
- To ensure correct payment for SSP claims, contractors should ensure that endorsements reflect the requirements outlined in the corresponding SSP endorsing guidance available on NHSBSA’s website.
- For SSPs that allow for an alternative product or quantity to be supplied, endorse the details of the alternative product(s) or quantity supplied in accordance with the SSP – not the details of the product or quantity that was originally prescribed.
- Only endorse strength(s)/formulation(s) of products that are covered by the SSP.
- Pharmacy teams are advised to familiarise themselves with PMR guidance about SSPs. Community Pharmacy England’s SSPs webpage endorsement section includes links to PMR-specific guidance.
- Contractors are strongly advised to check if their PMR system supports correct and complete SSP endorsements. If there is any doubt on the correct use of the SSP endorsement functionality available on PMR systems, contractors should contact their system supplier for further guidance. Any suggestions about how system usability and functionality could be improved should also be fed back to the system supplier.
- EPS tokens should not be used to make SSP claims. The electronic claim message created by the PMR system, including the electronic endorsements within it, is used to determine payment.
- Ensure your endorsement is accurate and clear – NHSBSA processing staff must be able to determine what has been supplied.
- If patient is exempt from prescription charges, the correct exemption reason should be selected.
- For patient’s that normally pay for their prescriptions, usual charges apply except for supplies in accordance with SSPs for a reduced quantity. No prescription charge is payable if a patient receives a smaller quantity of the medicine than the quantity originally ordered on the prescription if it was supplied in accordance with a SSP for a reduced quantity. If a patient normally pays for their prescriptions, the paid status should be selected on the EPS claim message. Once submitted for payment, the NHSBSA will recognise the SSP endorsement and process the prescription as a no-charge item i.e. no prescription charges will be deducted by the NHSBSA.
- Where applicable, check that the correct number of patient charges are collected and declared on the end of month FP34C submission form.
- If an electronic prescription has been endorsed incorrectly and before the claim is submitted for payment, where PMR systems allow, contractors may be able to retrieve any submitted EPS dispense notification messages by using the claim amend facility.
- SSPs cannot be supplied after the period of SSP validity. Any other items on the prescription (such as an owing) can continue to be dispensed as usual but must be submitted for payment within three months of expiry of the SSP.
Top Tips for HRT SSPs:
- Always double check that endorsements reflect the requirements outlined in the supporting guidance published for each SSP on the dedicated page of the NHSBSA’s website.
- Check whether your PMR system enables complete and correct SSP endorsements (please contact your system supplier if you are unsure of how to apply endorsements correctly). If you have suggestions about how system usability and functionality could be improved, feed these back to your PMR supplier.
- When endorsing an SSP for a reduced quantity in line with an SSP ensure you endorse the SSP product dispensed and the reduced quantity supplied.
- When endorsing an SSP for a substitution with a specific alternative product in line with an SSP ensure you endorse the specific alternative product dispensed and the quantity supplied.
- When endorsing an SSP for a reduced quantity and substitution in line with an SSP ensure you endorse the specific alternative product dispensed and the reduced quantity supplied.
- When endorsing an SSP for a substitution or a reduced quantity and substitution where a specific alternative brand has been dispensed contractors must endorse the brand supplied to ensure correct reimbursement. If a specific brand is supplied but the corresponding generic name is endorsed on the prescription, reimbursement would be in accordance with the price listed in Part VIIIA of the Drug Tariff which may result in underpayment.
- For SSPs involving substitution from gel/spray to patch formulation, or from Ovestin cream to Estriol 0.1% cream, there should not be a need to mark the prescribed product as ND (Not Dispensed). Please speak to your system supplier if you are unable to select and endorse a different product to what was originally prescribed.
- Ensure your endorsements are accurate and clear – NHSBSA processing staff must be able to determine what has been supplied.
- No prescription charge is payable if a patient receives a smaller quantity of the medicine than the quantity originally ordered on the prescription if it was supplied in accordance with a SSP for a reduced quantity. Prescription charges apply as usual for supplies made in accordance with SSPs permitting substitution only (without quantity restriction).
- If a patient normally pays for their prescriptions, the paid status should be selected even though no charge is taken for the three months’ worth of HRT medicine supplied in accordance with the SSP. Once submitted for payment, the NHSBSA will recognise the SSP endorsement and process the prescription as a no-charge item i.e. no prescription charges will be deducted by the NHSBSA.
- NHSBSA will continue to apply charge deductions for any other chargeable items dispensed on the same prescription form with mixed items (affected HRT medicine prescribed with other items).
- If patient is exempt from prescription charges, the correct exemption reason should be selected.
- If any claims for SSPs have been endorsed incorrectly and before a claim is submitted for payment, where PMR systems allow, contractors may be able to retrieve any submitted EPS dispense notification messages by using the claim amend facility.
- SSPs cannot be supplied past the period of SSP validity. Any other items on the prescription (such as an owing) can continue to be dispensed as usual but must be submitted for payment within three months of expiry of the SSP.
Top tips for Combisal®125mcg/25mcg inhalers SSP
- To claim the correct reimbursement and remuneration for supply in accordance with SSP034, follow the endorsing requirements as outlined in NHSBSA’s supporting guidance. See information on common SSPs endorsing errors identified by the NHSBSA.
- Contractors should check with their system supplier on the correct method of adding the required SSP endorsements including how to endorse a spacer device, if one is supplied.
- When a spacer device is supplied in accordance with SSP034, contractors should endorse the brand and type of spacer supplied for example, Volumatic® or Volumatic paediatric® with mask, if appropriate.
- If your PMR system does not allow endorsement of multiple products (inhaler and spacer) against a single prescribed item, contractors should endorse the alternative inhaler supplied along with the correct SSP endorsement. For the spacer device, the contractor would select the ‘NCSO’ endorsement and enter the brand/type of spacer supplied in the text.
- If a spacer cannot be added to endorsement this will not be reimbursed. If your PMR system functionality does not support correct endorsement requirements, the patient should be referred back to their prescriber to obtain a new prescription for an alternative inhaler and a compatible spacer.
| When supplying in accordance with these SSPs, contractors should refer to the updated endorsement guidance for each SSP available on NHSBSA’s SSP page. |
Community Pharmacy England’s support guidance to endorsing SSPs, click here.
NHSBSA’s support guidance to endorsing SSPs, click here.
Pharmacy system-specific guidance
Pharmacy system suppliers have informed Community Pharmacy England that system-specific SSP endorsing guidance will be made available and Community Pharmacy England has offered its support to suppliers in producing such guidance including guides or how-to videos. Guidance currently available includes:
- AAH ProScript Connect SSP guidance which links to sub-page: Using SSPs (updated Feb 2022)
- Analyst (Positive Solutions) SSP e-learning. The updated Help file for this system also includes SSPs information.*
- EMIS ProScript Connect SSP guide – also available on the EMIS support website.
- RxWeb (Clanwilliam) SSP guide
Notes:
- *Guidance with an asterix may not reflect the changes to endorsing requirements following the introduction of the new SSP endorsement from 1st June 2021.
- Note: Other suppliers not listed above should also provide guidance for the users of their system.
- It is recommended that contractors take up opporunities to update their system where this is recommended by the supplier in case later versions have improvements or new functionalities – including in relation to SSPs
- Contractors using the same system may be on different versions and guidance will show a specific version.
Please speak to your system supplier for the latest system-specific SSP endorsing guidance, or if you suggestions about how to improve the SSP functionality available within your system.
Read more about how to request guidance or improved features: Making your system work for you and Feeding back or reporting about EPS.
- Select the SSP endorsement.
- Input the appropriate 3-digit reference number of the SSP.
- Follow the specific supporting endorsement guidance for the applicable SSP – often this will require contractors to select and endorse the SSP product and quantity supplied to the patient.
- Contractors should check with their system supplier on the correct use of the SSP endorsement functionality particularly where the dispensed item is different to the prescribed item. Contractors should note that only the correct endorsements contained in the electronic message are used to price electronic prescriptions (i.e. handwritten endorsements on the corresponding EPS Tokens are not referred to by the NHSBSA when pricing SSP claims).
- Endorse SSP and the appropriate 3-digit reference number
- Follow the specific supporting endorsement guidance for the applicable SSP – often this will require contractors to endorse the relevant SSP product dispensed including strength, pharmaceutical form, and quantity supplied to the patient.
- Place the prescription in the red separator.
To ensure correct reimbursement, pharmacy teams should ensure that all Electronic Reimbursement Endorsement Messages (EREMs) or EPS claim notification messages and/or paper FP10 forms with SSP claims are submitted to NHSBSA in a timely manner.
If SSP claims are submitted electronically, the Dispense Notification (DN) message should be submitted during the period of SSP validity. Pharmacy teams should take into account the EPS 5 day window and EPS submission timing when sending the EREMs i.e. submit claim message no later than the 5th of the month following supply. Any paper prescriptions with SSP claims must be placed in a red separator and dispatched to the relevant division of the NHSBSA by the 5th of the month following that in which supply was made.
Submission of prescriptions containing both SSP(s) and non-SSP(s)
Contractors have to submit claims for SSPs in the usual manner, i.e. by the 5th of the month following that in which supply was made . However, any prescriptions with mixed (SSP and non-SSP items) may be claimed up to three months after the SSP expires to help deal with claims for any owings on the prescription. Using the example of SSP 009 which expired 17 May 2021, the final cut-off for submission to be reimbursed for this SSP would be with your July bundle (submitted by the 5th of August).
Please note that SSPs cannot be supplied past the period of SSP validity. Any other items on the prescription (such as an owing) can continue to be dispensed as usual but must be submitted for payment within three months of expiry of the SSP.
Declaration of monthly SSP claims using the FP34C form on MYS
- There is a separate declaration of monthly SSP claims on the digital FP34C submission form via the Manage Your Service (MYS) portal for contractors to declare the correct number of SSP claims made each month.
- Separately, contractors should also include the number of items supplied in accordance with SSPs with the usual item totals.
- Please note that the NHSBSA will not be using the SSP declaration made on the FP34Cs for reimbursement purposes. Instead, the SSP declaration is to provide the NHSBSA with an indication that SSP claim(s) are expected in that month
| All paper FP10 forms should be placed in the front of the red separator for the end-of-month submission bundle |
- Incorrect dispensed item endorsed – for SSPs that require substitution to an alternative product, the item endorsed was very often the same as original prescribed item.
- Claims made against items without an active SSP – SSP endorsement applied against items for which a valid SSP does not exist.
- Reduced quantity not endorsed– for SSPs that require a reduced quantity to be endorsed, often the endorsed quantity matched the prescribed quantity.
- Claims for supplies made in accordance with an expired SSP:
- No active SSP in place at the time of supply – i.e., SSP claimed for items after expiry of SSP.
- Alternative item correctly supplied during the period of SSP validity, but Dispense Notification message submitted after the period of SSP validity.
- Alternative item correctly supplied during the period of SSP validity and the Dispense Notification message submitted during the period of SSP validity but the EREM or Claim Notification message submitted after the 3-month claiming window (where multiple items are ordered on the same prescription).
- Invalid or incorrect SSP reference number endorsed – The endorsed SSP number does not match the actual SSP number. For example, SSP470 (this SSP number does not exist); SSP(blank) (missing reference number); and SSP47 (two instead of three digits).
We recommend pharmacy teams spot check the number of SSPs performed and declared versus the number NHSBSA priced (using these steps).
These are outlined within a one-page factsheet and are also seen below:
1. Use endorsing guidance: Use Community Pharmacy England SSP endorsing guidance and your Patient Medical Record system specific guidance (queries to suppliers about SSPs can be raised in the usual way). Ensure that SSP endorsements are only added if the relevant criteria is fulfilled.
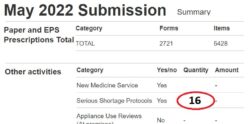 2. Tally your SSP totals: Tally the genuine SSPs carried out, as you do these. This tally could differ from your PMR total (if your team or system inadvertently endorses SSP without intending to carry out an SSP). NHSBSA will only price valid SSPs.
2. Tally your SSP totals: Tally the genuine SSPs carried out, as you do these. This tally could differ from your PMR total (if your team or system inadvertently endorses SSP without intending to carry out an SSP). NHSBSA will only price valid SSPs.
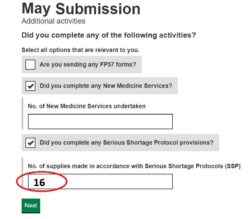
3. Submit your SSP total: Enter onto your monthly submission the tally number of SSPs (see ‘Figure 1’) on the Manage Your Service (MYS) portal.
4. Digitally file your MYS confirmation email and records of totals submitted: This is saved into your MYS portal history (see ‘Figure 2’).
 5. Await pricing of the month and compare priced to declared totals: Await your Schedule of Payment and after receipt of it compare totals. The priced totals are in the ‘Details of Other Amounts Authorised’ section typically
5. Await pricing of the month and compare priced to declared totals: Await your Schedule of Payment and after receipt of it compare totals. The priced totals are in the ‘Details of Other Amounts Authorised’ section typically
found on page 2 or 3 (see ‘Figure 3’). Check the priced totals compared to your MYS email totals.
6. Spotting a discrepancy: If you can identify specific SSPs, check whether these will have gone into the dispensing month in question (further info: EPS submission timing). Consider whether the SSPs in question were endorsed correctly – you can view the previous prescriptions within your PMR. If you suspect the PMR system supplier needs to be made aware of an issue, use the PMR escalation route.
7. Still worried? If there is a payment discrepancy and it is believed that the correct SSP endorsing guidance was followed, pharmacy owners may consider requesting a re-check of relevant prescriptions and the NHSBSA will be able to either rectify the payment or explain why the claim was unpaid incorrectly or if appropriate to rectify the payment (cpe.org.uk/recheck). We have not yet had evidence that valid EPS SSP endorsements have gone ‘unpriced’.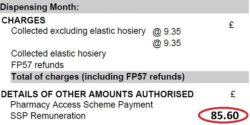
SSPs and VAT
The Department of Health and Social Care (DHSC) has provided clarification from HMRC that VAT is not applicable on products supplied in accordance with serious shortage protocols (SSPs). From 1 June 2023, the allowance for VAT currently paid alongside product reimbursement will stop being paid when reimbursing for products supplied in accordance with SSPs. Until the end of May 2023, for products supplied in accordance with a SSP, a VAT allowance will continue to be paid. This change will be reflected from the June 2023 Drug Tariff.
This change in VAT status for SSPs was initially communicated by Department of Health and Social Care (DHSC) and NHS England in the letter to community pharmacy owners published on 12 May 2023. Our members will need to reclaim VAT paid on the purchase of medicines supplied in accordance with an SSP, as they ordinarily do for medicines dispensed against prescriptions. HMRC will be updating their guidance on the correct VAT liability of SSP shortly.
Q. When may supply in accordance with an SSP be refused?
A. Contractors may refuse to supply an alternative product or quantity in accordance with the SSP, if the supervising pharmacist considers that supply of the different or alternative product or quantity is unreasonable or inappropriate. Contractors must refuse to supply an alternative product or quantity in accordance with the SSP, if such a supply has already been made in accordance with the presented prescription.
Q. Do the SSPs for apply to private prescriptions?
A. Yes. The scope of the SSPs applies to valid prescriptions that meets the requirements of the Human Medicine Regulations 2012, so it would cover both NHS and private prescriptions, unless where it stated otherwise on the SSP itself.
Q. Do patients have to consent/agree to SSP supplies?
A. Yes, patient consent/agreement is required for supply of an alternative product or quantity in accordance with an SSP.
Q. Do patients pay prescription charges for SSP supplies?
A. Generally, prescription charges are payable for the supply of alternative products in accordance with SSPs, if they were payable for supply under the original prescription. The exception to this is that no prescription charge is payable if the patient is supplied with a smaller quantity of drug or fewer appliances than originally prescribed. This exception was proposed by Community Pharmacy England and accepted by DHSC Ministers in order that patients that pay the prescription charge are not disadvantaged by the use of an SSP.
Q. Do prescription charges apply for quantity restriction SSPs?
A. No prescription charge is payable if a patient receives a smaller quantity of the medicine than the quantity originally ordered on the prescription if it was supplied in accordance with an SSP for reduced quantity.
If patient is exempt from prescription charges, the correct exemption reason should be selected.
If a patient normally pays for their prescriptions, the paid status should be selected even though no charge is taken. Once submitted for payment, the NHSBSA will recognise the SSP endorsement and process the prescription as a no-charge item i.e. no prescription charges will be deducted by the NHSBSA.
NHSBSA will continue to apply charge deductions for any other chargeable items dispensed on the same prescription form with mixed items.
Q. If the prescribed item cannot be obtained after the expiry of an SSP, can I continue to make supplies of the alternative(s) specified in the expired SSP until stock of the prescribed item becomes available?
A. No. Once an SSP has expired/been withdrawn it is no longer valid for use and you must dispense in accordance with the prescription. If the prescribed item remains unavailable, the patient should be referred back to the prescriber for an alternative.
Q. Do the other provisions of the terms of service apply to SSP supplies?
A. Yes. SSPs are now part of the Essential Dispensing Service, part of Schedule 4 of the NHS Pharmaceutical Regulations and, broadly, the terms of service apply; including, for example, that contractors must provide appropriate advice as required and maintain appropriate records.
Specific amendments in the NHS (Amendments Relating to Serious Shortage Protocols) Regulations 2019, to ensure the terms of service are relevant to SSPs, include:
- Measuring and fitting for appliances (Reg. 8 (4))
- Relevant standards and formula (Reg. 8 (5))
- Original pack dispensing (Reg. 8 (10))
- Suitable containers (Reg. 8 (15))
- Alternative to referral for an appliance (Reg. 10 (2)(b)
Q. When endorsing a prescription for an SSP, does the SSP number need to be included?
A. Yes. When endorsing using EPS, select the SSP endorsement on your system and input the correct three-digit reference number. If present, remember to insert the leading zero in the three-digit reference number for example SSP022.
Q. When endorsing a prescription for an SSP do I have to endorse the pack size?
A. Pharmacy teams should endorse the prescription in line with the endorsement requirements specified in the protocol which may indicate whether a pack size endorsement is required.
Q. What are the correct requirements for SSP endorsements?
A. Pharmacy teams are advised to refer to the individual protocol for specific endorsement requirements. Pharmacy teams are required to endorse the prescription with ‘SSP’ to indicate that a supply was made in accordance with an SSP. The use of the SSP endorsement will indicate to the NHSBSA that an SSP was used, i.e. that the originally prescribed product was not dispensed by the pharmacy and a different quantity or product was supplied in accordance with an SSP.
To ensure correct payments (fees and reimbursement) for products supplied under an SSP, pharmacy teams are generally required to endorse the following information on the paper prescription or where issued using EPS, using either the original electronic prescription (as outlined in Part II of the Drug Tariff):
- SSP (followed by the three-digit reference number applicable to the SSP) – to indicate that the supply was made in accordance with an SSP;
- Details of product supplied in accordance with the SSP (drug name, quantity, strength, formulation, supplier name or brand) *;
- Quantity supplied*;
- Pack size – best practice where multiple pack sizes are available; and
- Invoice price – only if NHSBSA has no list price available on dm+d.
* The drug details and quantity supplied and endorsed will depend on the specific SSP.
Q.I have received a prescription for ‘Drug X’ 28 tablets. I only have 14 tablets in stock, however, there is a valid SSP in place for ‘Drug X’ allowing substitution of tablets to capsules. Can I dispense the 14 tablets of ‘Drug X’ that I have in stock and use the SSP to supply the remaining balance as capsules?
A. No. Should you wish to use the stock you currently have on your shelf you should dispense the prescription as per usual processes, clearly endorsing the quantity to show that 14 tablets only were dispensed. Alternatively you can consider supply of the capsules in accordance with the SSP for ‘Drug X’ .
Q. I have a prescription for two items; one item covered by an active SSP and the other for a non-SSP item. The item covered by SSP is supplied within the period of SSP validity but the non-SSP item is only part-dispensed with an owing for the patient. The balance of the non-SSP item is not dispensed to the patient until after the expiry of the SSP. Will I be reimbursed for an SSP if the prescription is submitted for payment after the SSP expiry date?
A. Pharmacy teams have to submit their claim for the SSPs in the usual manner, i.e. by the 5th of the month following that in which supply was made. However, any prescriptions with mixed (SSP and non-SSP items) may be claimed up to three months after the SSP expires to help deal with claims for any owings on the prescription. Using the example of SSP01 which expired 25 October, the final cut-off for submission to be reimbursed for this SSP would be with your December bundle (submitted by the 5th of January).
Please note that SSPs cannot be supplied past the period of SSP validity. Any other items on the prescription (such as an owing) can continue to be dispensed as usual but must be submitted for payment within three months of expiry of the SSP.
Q. How can I ensure that the paper prescriptions with SSP claims are seen by an exception handler at NHSBSA?
A. All paper prescriptions which SSP claims must be placed in the red-separator on top of the bundle before submitting to the NHSBSA in the usual way.
Q. If a prescription with an SSP claim is not endorsed with a formulation, quantity or strength, would the prescription (or a copy of) be returned to the contractor for clarification?
A. Where insufficient information is available to enable the NHSBSA to process an SSP claim, the prescription or shall be returned to the contractor to endorse the prescription form (or copy of the original form) with the information requested. Returned prescriptions shall be priced using the Drug Tariff relevant to the month in which the prescription form (or copy) is returned to the NHSBSA. If the initials ‘SSP’ are missing on any SSP claim, the prescription will not be returned to the contractor and any reimbursement will be based on the original prescribed order.
Q. I have submitted a prescription with an SSP claim to NHSBSA but omitted the endorsement of ‘SSP’; will NHSBSA return the prescription to us for clarification?
A. No. To be reimbursed for supplies made against an SSP, the endorsement ‘SSP’ must be present. Failure to endorse ‘SSP’ would result in the prescription being reimbursed based on the original prescribed order.
Q. How will payments for SSPs claimed appear on my Schedule of Payments?
A. The Schedule of Payment will include two single lines – one for total VAT and other for fees (if applicable).
Q. Do the SSPs for HRT apply to cross-border prescriptions?
A. Yes. Patients from England, Scotland, Wales or Northern Ireland who present their prescriptions for either Premique® Low Dose 0.3mg/1.5mg modified-release tablet, Ovestin® 1mg cream, Oestrogel® Pump-Pack 0.06% gel, Lenzetto® 1.53mg/dose transdermal spray or Sandrena® 0.5mg and 1mg gel sachets are eligible to receive supply under the terms of the specific appropriate SSP.
Q. Do patients have to consent/agree to SSP supplies?
A. Yes, patient consent/agreement is required for supply of an alternative product and/or quantity in accordance with an SSP.
Q. Do the SSPs for HRT apply to private prescriptions?
A. Yes. The scope of the SSPs for HRT applies to valid prescriptions that meets the requirements of the Human Medicine Regulations 2012, so it would cover both NHS and private prescriptions, unless where it stated otherwise on the SSP itself.
Quantity restriction SSPs
Q. Will prescription charges apply for quantity restriction SSPs?
A. No prescription charge is payable if a patient receives a smaller quantity of the medicine than the quantity originally ordered on the prescription if it was supplied in accordance with an SSP for reduced quantity.
If patient is exempt from prescription charges, the correct exemption reason should be selected.
If a patient normally pays for their prescriptions, the paid status should be selected even though no charge is taken for the three months’ worth of HRT medicine supplied in accordance with the SSP. Once submitted for payment, the NHSBSA will recognise the SSP endorsement and process the prescription as a no-charge item i.e. no prescription charges will be deducted by the NHSBSA.
NHSBSA will continue to apply charge deductions for any other chargeable items dispensed on the same prescription form with mixed items (affected HRT medicine prescribed with other items).
Q. How would pharmacists determine a three-month supply?
A. Where it is not clear from the prescription what constitutes a three-month supply, the pharmacist will need to discuss with the patient and use their professional judgment.
Q. If the prescription states less than three months’ supply should be dispensed does it fall within scope of a quantity restriction SSP?
A. No. If the prescription states that either three months’ supply or less is to be supplied to the patient, this would not fall within scope of a quantity restriction SSP. The pharmacist should dispense the medication in accordance with the prescription.
Substitution SSPs
Q. Will prescription charges apply for substitution SSPs?
A. Yes, for prescriptions with a quantity less than three-months, patients who are not exempt from prescription charges would continue to pay for this as usual.
Q. Can pharmacists supply more than three months’ supply for a substitution SSP?
A. No, in accordance with the substitution SSPs, pharmacists will only be able to dispense the substituted product when the prescribed duration of treatment is three months’ or less.
Q. Is there any difference between the substituted products recommended?
A. Yes. Pharmacists should be mindful of the different dosing regimens of the substituted products specified on the supporting guidance. For example, whether the patches are administered once or twice weekly.
Pharmacists should use their professional judgement to select the appropriate substitution on the basis of the brands available to them.
Q. How will I be reimbursed when supplying a substitution with a specific alternative product in line with an SSP?
A. Reimbursement will be for the the medicine supplied in accordance with an SSP, not the originally prescribed medicine.
Where a specific alternative brand has been dispensed contractors must endorse the brand supplied to ensure correct reimbursement. If a specific brand is supplied but the corresponding generic name is endorsed on the prescription, reimbursement would be in accordance with the price listed in Part VIIIA of the Drug Tariff which may result in underpayment.
Substitution AND quantity restriction SSPs
Q. Will prescription charges apply for substitution and quantity restriction SSPs?
A. No prescription charge is payable if a patient receives a smaller quantity of the medicine than the quantity originally ordered on the prescription if it was supplied in accordance with an SSP for reduced quantity.
If patient is exempt from prescription charges, the correct exemption reason should be selected.
If a patient normally pays for their prescriptions, the paid status should be selected even though no charge is taken for the three months’ worth of HRT medicine supplied in accordance with the SSP. Once submitted for payment, the NHSBSA will recognise the SSP endorsement and process the prescription as a no-charge item i.e. no prescription charges will be deducted by the NHSBSA.
NHSBSA will continue to apply charge deductions for any other chargeable items dispensed on the same prescription form with mixed items (affected HRT medicine prescribed with other items).
Q. Is there any difference between the substituted products recommended?
A. Yes. Pharmacists should be mindful of the different dosing regimens of the substituted products specified on the supporting guidance. For example, whether the patches are administered once or twice weekly.
Pharmacists should use their professional judgement to select the appropriate substitution on the basis of the brands available to them.
Q. How would pharmacists determine a three-month supply?
A. Where it is not clear from the prescription what constitutes a three-month supply, the pharmacist will need to discuss with the patient and use their professional judgment.
Q. If the prescription states less than three months’ supply should be dispensed does it fall within scope of a substitution and quantity restriction SSP?
A. No. If the prescription states that either three months’ supply or less is to be supplied to the patient, this would not fall within scope of a substitution and quantity restriction SSP. The pharmacist should dispense the medication in accordance with the prescription.
Q. How will I be reimbursed when supplying a substitution with a specific alternative product in line with an SSP?
A. Reimbursement will be for the the medicine supplied in accordance with an SSP, not the originally prescribed medicine.
Where a specific alternative brand has been dispensed contractors must endorse the brand supplied to ensure correct reimbursement. If a specific brand is supplied but the corresponding generic name is endorsed on the prescription, reimbursement would be in accordance with the price listed in Part VIIIA of the Drug Tariff which may result in underpayment.
If and when an SSP is revoked/expired, it will be removed from the live table above and instead placed below for historical reference.












