How the price change mechanism works
Published on: 17th July 2013 | Updated on: 8th December 2023
As prices in the market are constantly changing, there is an agreed mechanism in place to increase and decrease the reimbursement prices for medicines. This is intended to take into account pharmacies having stock of certain products already on their shelves when a price change is first introduced.
Community Pharmacy England keeps the proprietary and non-proprietary price change mechanisms under close review. It is expected that the price change mechanism would have a neutral effect on pharmacy contractors’ reimbursement given that prices decrease as well as increase from time to time.
Download our Factsheet: What is the Price Change Mechanism?
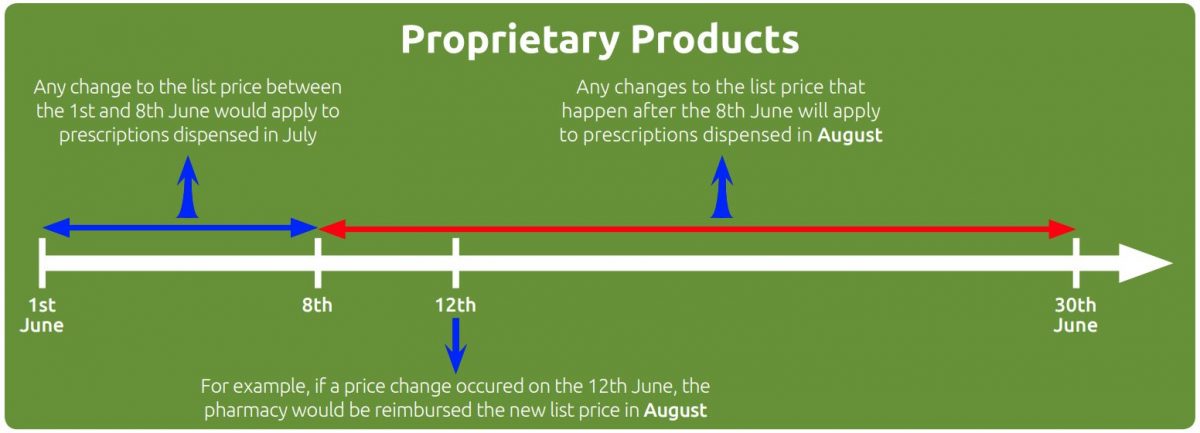
Proprietary products
For proprietary preparations and Part VIIIA products where the price is based on a proprietary product (e.g. Part VIIIA Category C products such as Dalteparin sodium 10,000units/1ml solution for injection ampoules based on Fragmin), a price change up to and including the 8th of the month takes effect for prescriptions dispensed in the following month. A price change after the 8th of the month will be applied for reimbursement purposes to prescriptions dispensed one month later.
For example, if the manufacturer’s list price for a proprietary product changed on the 6th of June, the new reimbursement price would apply to prescriptions dispensed in July. If a manufacturer’s list price changed on the 15th June, the new reimbursement price would apply to prescriptions dispensed in August.
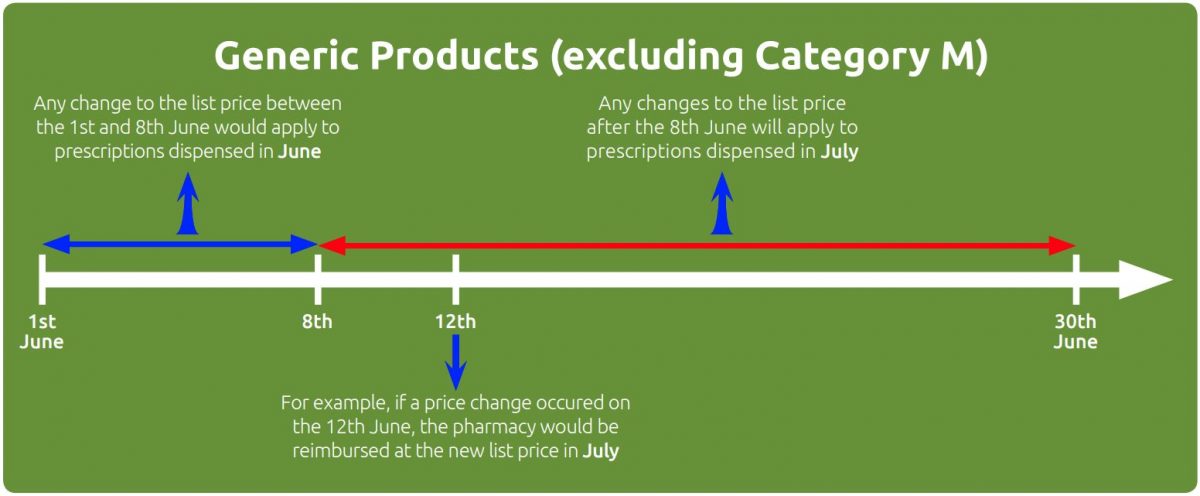
Generic products
For non-proprietary or generic products (excluding products in Part VIIIA, Category M) the reimbursement price change takes place one month earlier than proprietary products. For example, if the manufacturer’s list price changed on the 6th of June, the new reimbursement price would apply to prescriptions dispensed in June. If a manufacturer’s list price for a generic drug changed on the 15th June, the new reimbursement price would apply to prescriptions dispensed in July.
Part VIIIA Category A includes popular generics, which are readily available. The price is based on a weighted average of the List Prices from 2 wholesalers and 2 generic manufacturers.
Category M products
Drugs listed as Category M in the Drug Tariff are not affected by the Price Change Mechanism.
Part VIIIA Category M includes drugs that are readily available. Category M prices are provided by the Department of Health and Social Care (DHSC) each quarter. As DHSC are aware of the price change timetable and the publication deadlines, the prices are received and incorporated in the Drug Tariff prior to the 15th of the month.
If contractors are unable to purchase a Category M drug at the Drug Tariff listed price, these should be reported to the Community Pharmacy England Dispensing and Supply Team using our online feedback form (cpe.org.uk/feedback). Community Pharmacy England will investigate the extent of the problem and, where necessary apply for a price concession.
More information on Category M price changes is available here: cpe.org.uk/categorym
For more information on price concessions, see our page: cpe.org.uk/priceconcessions