MHRA Class 4 Medicines Defect Information: Briviact 75mg & 100mg film-coated tablets (Ethigen Limited)
Class 4 Medicines Defect Information:
Briviact 75mg & 100mg film-coated tablets (Ethigen Limited)
Drug alert number: EL (23)A/13
Date issued: 30 March 2023
The Medicines and Health products Regulatory Agency (MHRA) has issued a class 4 medicines defect information notice for:
Briviact 75 mg film-coated tablets, PLPI 18716/0146
SNOMED Code: 37622711000001100
| Batch number | Expiry date | Pack size | First distributed |
| 340132 | 30/09/2024 | 56 | 15-Nov-22 |
| 332075 | 28/02/2025 | 56 | 06-Dec-22 |
Active Pharmaceutical Ingredient: brivaracetam
Briviact 100 mg film-coated tablets, PLPI 18716/0147
SNOMED Code: 37622711000001101
| Batch number | Expiry date | Pack size | First distributed |
| 332076 | 28/02/2025 | 56 | 09-Mar-22 |
| 329941 | 31/12/2024 | 56 | 09-May-22 |
| 337225 | 30/04/2025 | 56 | 30-May-22 |
| 332072 | 31/03/2025 | 56 | 01-Jun-22 |
| 329491 | 31/12/2024 | 56 | 08-Jun-22 |
| 337216 | 31/05/2025 | 56 | 09-Jun-22 |
| 339975 | 30/06/2025 | 56 | 27-Jun-22 |
| 337216 | 31/05/2025 | 56 | 29-Jul-22 |
| 343194 | 30/09/2025 | 56 | 14-Nov-22 |
| 349497 | 31/10/2025 | 56 | 16-Nov-22 |
| 343190 | 30/09/2025 | 56 | 12-Dec-22 |
| 349527 | 30/11/2025 | 56 | 14-Feb-23 |
Active Pharmaceutical Ingredient: brivaracetam
Brief description of the problem
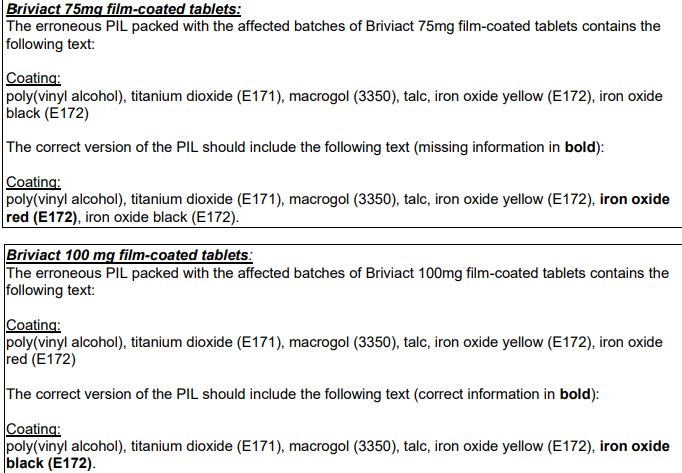
Ethigen Limited have informed MHRA that due to a formatting error of the Patient Information Leaflet (PIL), incorrect and missing information about the colorant ingredients of the tablet coating was listed in Section 6 of the PIL:
Advice for healthcare professionals
There is no risk to product quality as a result of this issue. Healthcare professionals are advised to exercise caution when dispensing the above batches of the product. Where possible, please provide an updated copy of the PIL to the patient and remind the patient to read the leaflet in its entirety before using the medicine. These are available on the MHRA website by entering the product licence (PLPI) numbers. Alternatively, these may also be accessed via the links below:
Briviact 75mg film-coated tablets
Briviact 100mg film-coated tablets
Upon request, Ethigen Limited will post hard copies of the updated PIL to wholesalers and pharmacies so that any remaining stock in the dispensary can be supplemented with the correct PIL information.
View full alert here.
Advice for patients
This notification relates to a typographical error in the Patient Information Leaflet (PIL) relating to the ingredients of the tablet coating of the medicine. Patients do not need to take any action as the medicine itself is not affected. Any suspected adverse reactions should be reported via the MHRA Yellow Card scheme.
Further Information
For medical information and stock control queries please contact the Regulatory Affairs Department at regulatory@ethigen.co.uk